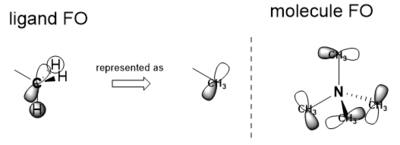
Rep:Mod:im3117
im3117 Inorganic Wiki Page
BH3 Molecule
Analysis
Settings: B3LYP/6-31G(d,p)
Table 1
Item Value Threshold Converged? Maximum Force 0.000193 0.000450 YES RMS Force 0.000126 0.000300 YES Maximum Displacement 0.000764 0.001800 YES RMS Displacement 0.000500 0.001200 YES
Table 2
Low frequencies --- -0.2261 -0.1036 -0.0054 48.0227 49.0824 49.0830 Low frequencies --- 1163.7223 1213.6713 1213.6740
Frequency file: BH3_Frequency.log
Figure 1
optimised BH3 molecule |
Lowest frequency is outside the suggested ± 15 cm-1 range. This can be the case for small molecules as the frequencies can be attributed to the basicity of the basis set and the relatively relaxed convergence and integration criteria.
Vibrational spectrum for BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1164 | 92 | A2 | yes | out-of-plane bend |
| 1214 | 14 | E' | very slight | in-plane bend |
| 1214 | 14 | E' | very slight | in-plane bend |
| 2580 | 0 | A'1 | no | totally symmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
Figure 2
There are fewer vibrational peaks than vibrations as not all of the vibrations are IR active. To satisfy the selection rules for IR activity, a bend/stretch must result in a change in dipole moment. As the symmetric stretch (2580 cm-1) does not have a change in dipole moment, it is not visible on the spectrum.
Ng611 (talk) 20:21, 29 May 2019 (BST) This accounts for one missing peak, but what about the other missing peaks?
PP and Basis Sets
Table 4
A table produced by Gaussian indicating the features of the optimised BH3 molecule.
MO Diagram
Figure 3
A qualitative MO diagram indicating the predicted ordering of the BH3 orbitals. The 1a1 ' is too low in energy to interact with the a1' symmetry atomic orbitals from the H3 fragment. It therefore solely comprises boron atomic orbitals. 2a1' has larger contribution from H3 as the molecular orbital is closer in energy to its atomic orbitals. The 1e' orbitals are low in energy due to the in-phase overlap between the atomic orbitals, producing a frontier orbital with a larger contribution from H3 due to it being closer in energy. 1a2 is a non-bonding orbital as there are no other atomic orbitals with the same symmetry for it to interact with. As there is no interaction, the orbitals is neither raised nor lowered in energy. The 3a1' orbital has a larger contribution from the boron 2s orbital as it is closer in energy to this frontier orbital. It is more destabilised than the 2a1' is stabilised. The 2e' orbitals are anti-bonding molecular orbitals as they contain out-of-phase overlap with greater contribution from boron's 2p orbitals as they are higher in energy and therefore closer in energy. The relative ordering of 3a1 ' and 2e' is difficult to ascertain as s-s interactions are stronger, however a1' are lower than e'.
Ng611 (talk) 20:22, 29 May 2019 (BST) Good!
Real MOs
| MO (incl. symmetry) | Energy (eV) | Picture | Theory Prediction | Filled? |
| 1a1' | -6.77 | 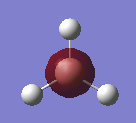 |
 |
Y |
| 2a1' | -0.51 | 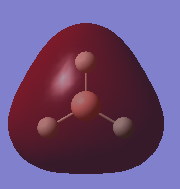 |
 |
Y |
| 1e' | -0.35 | 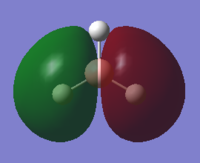 |
 |
Y |
| 1e' | -0.35 | 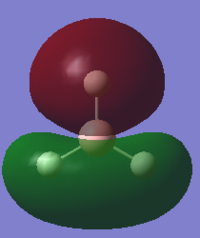 |
 |
Y |
| 1a2 | -0.07 | 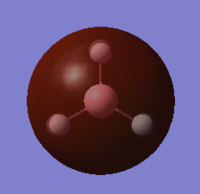 |
 |
N |
| 3a1' | 0.17 | 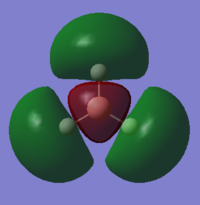 |
 |
N |
| 2e' | 0.18 | 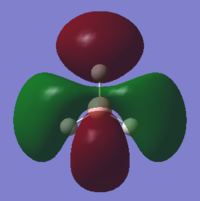 |
 |
N |
There are no significant differences between the real and LCAO MOs. The LCAO MOs allow the prediction of the number of nodes and therefore the bonding or antibonding character of the orbital. The shape of the orbitals is somewhat harder to predict however they do fall in line with the LCAO predictions in terms of phases. Qualitative MO theory is very useful and accurate however some flaws can exist when interpreting the energetic ordering of some of the frontier molecular orbitals. This is because it can be hard to quantify the interactions between orbitals and which effect has greater importance in a specific instance. For example, in the MO diagram, as seen in Figure 3, the prediction of the ordering of 3a1' versus 2e' was hard to quantify.
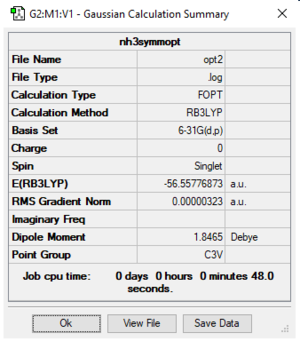
NH3
Analysis
Settings: B3LYP/6-31G(d,p)
NH3 optimised bond length = 1.02 Å ; H-N-H optimised bond angle = 105.7 °
Table 6
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Table 7
Low frequencies --- -8.5646 -8.5588 -0.0044 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Frequency file: NH3_Frequency.log
Table 8
A table produced by Gaussian indicating the features of the optimised NH3 molecule.
Figure 4
optimised NH3 molecule |
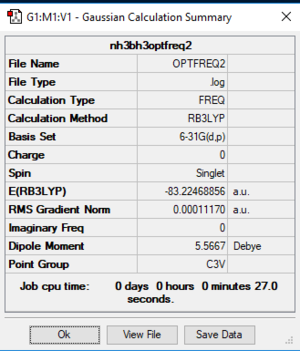
NH3BH3
Analysis
Settings: B3LYP/6-31G(d,p)
Optimised bond lengths: B-N = 1.67 Å; B-H = 1.21 Å; N-H = 1.02 Å
Optimised bond angles: H-B-H = 113.9 °; H-N-H = 107.9 °
Table 9
Item Value Threshold Converged? Maximum Force 0.000233 0.000450 YES RMS Force 0.000083 0.000300 YES Maximum Displacement 0.001202 0.001800 YES RMS Displacement 0.000370 0.001200 YES
Table 10
Low frequencies --- -0.0277 -0.0069 -0.0053 10.0759 10.1235 37.9189 Low frequencies --- 265.3049 634.4304 639.2081
Frequency file: NH3BH3_Frequencies.log
Table 11
optimised NH3BH3 molecule |
Energies
1 a.u. = 627.5 kcal/mol 1 a.u. = 2625 kJ/mol
E(NH3)= -56.55777 au (to 5 dp)
E(BH3)= -26.61532 au (to 5 dp)
E(NH3BH3)= -83.22469 (to 5 dp)
ΔE = E(NH3BH3)-[E(NH3)+E(BH3)] = -83.22469 - [-56.55777-26.61532] = 0.0516 au = 135 kJmol-1 (to nwn)
Based on my energy calculation is the B-N dative bond weak, medium or strong? By comparing the electronegativities of B and N, 2.04 and 3.04 respectively, the B-N bond is a polar covalent bond but the charge density is not incredibly separated[1]. The elements also occupy the same period of the periodic table meaning there is increased overlap between the orbitals, strengthening the bond. The lone pair on the NH3 also quenches the Lewis acidity of the BH3, arising due to the electron deficiency in the empty p orbital. All of these factors suggest that the B-N is a medium strength bond. However in comparison with the isoelectronic C-C bond, 346 kJmol-1, it is weaker[2]. Compared with a N-N bond, whose bond strength is 167 kJmol-1, the bond is weaker. The decreased bond strength can be attributed to the dative nature of the bond as the electrons both come from the nitrogen lone pair and are donated into the boron's empty p orbital.
Ng611 (talk) 20:24, 29 May 2019 (BST) Excellent!
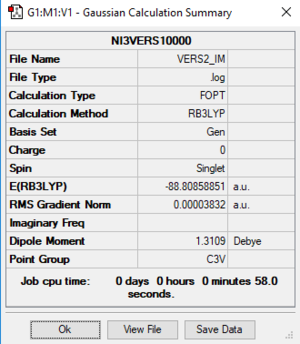
NI3 Molecule
Analysis
Settings: B3LYP/6-31G(d,p)LANL2DZ
Optimised N-I bond distance = 2.18 Å
Ng611 (talk) 20:25, 29 May 2019 (BST) Remember to report your bond values to 3 d.p.
Table 12
Table 13
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000044 0.000300 YES Maximum Displacement 0.000492 0.001800 YES RMS Displacement 0.000333 0.001200 YES
Table 14
Low frequencies --- -12.3847 -12.3783 -5.6131 -0.0040 0.0194 0.0711 Low frequencies --- 100.9307 100.9314 147.2333
Frequency file: NI3_Frequency.log
Figure 4
optimised NI3 molecule |
[N(CH3)4]+
Analysis
Settings: B3LYP/6-31G(d,p)
Table 15
Item Value Threshold Converged? Maximum Force 0.000073 0.000450 YES RMS Force 0.000018 0.000300 YES Maximum Displacement 0.000277 0.001800 YES RMS Displacement 0.000088 0.001200 YES
Table 16
Low frequencies --- 0.0008 0.0009 0.0011 35.6259 35.6259 35.6259 Low frequencies --- 215.5175 315.1186 315.1186
Frequency file: N(CH3)4+_Frequency.log
These zero frequencies are higher than anticipated however is sufficient for this purpose. These values occur because the basis set used is insufficient to detect some of the more subtle vibrational modes.
Table 17
Figure 5
optimised NH3BH3 molecule |
MOs
Note: for MOs 16-21 and 23-26, more significant figures were given to provide distinction between non-degenerate levels.
MO Analysis
Ng611 (talk) 20:28, 29 May 2019 (BST) Excellent LCAO analysis. I'd make your notes a little more detailed though.
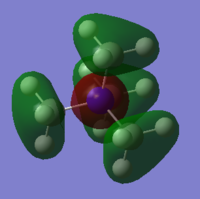
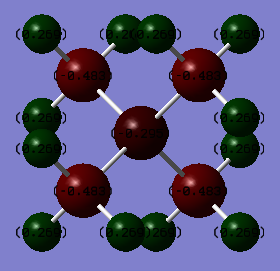
Charge Density
Figure 6
| Atom (in [N(CH3)4]+) | Charge (C) |
| N | -0.295 |
| C | -0.483 |
| H | 0.269 |

Typically, NR4+ is portrayed as seen in Figure 7, which is supported by the charge distribution analysis seen in the table above, Table 20. This positive charge is a Lewis charge, arising from the fact that the nitrogen uses its Lewis basic lone pair of electrons and donates them to form a bond with an alkyl group. It also arises from the fact that nitrogen only has 3 unpaired electrons and therefore would expect to form only three bonds. This extra bond removes some of the electron density from the nitrogen atom, leaving it slightly positive. The nitrogen's positive charge would most likely be spread over the other electropositive atoms in the system, such as the hydrogen. As the alkyl groups are electron-donating, this positive charge on the nitrogen, arising from slight electron deficiency, will be stabilised.
Ng611 (talk) 20:31, 29 May 2019 (BST) I'm confused, the calculated nitrogen charge is negative, not positive. This is in complete disagreement with Lewis bonding. Remember that you're being asked to compare the calculation to the formal bonding picture, which predicts an N-localised positive charge.
[P(CH3)4]+
Analysis
Settings: B3LYP/6-31G(d,p)
Table 21
Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.000666 0.001800 YES RMS Displacement 0.000277 0.001200 YES
Table 22
Low frequencies --- -0.0036 -0.0009 -0.0002 51.2698 51.2698 51.2699 Low frequencies --- 186.5950 211.3905 211.3905
Frequency file: P(CH3)4+_Frequencies.log
Table 23
optimised NH3BH3 molecule |
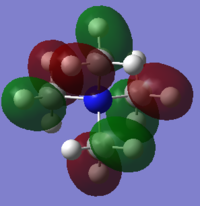
Charge Analysis
Figure 8
| Atom (in [P(CH3)4]+) | Charge (C) |
| P | 1.666 |
| C | -1.060 |
| H | 0.298 |
Comparisons
| [N(CH3)4]+ Charge Distribution | [P(CH3)4]+ Charge DIstribution |
 |

|
The same colour range (the largest out of both) were used to visualise both molecules, as seen below in Figure 9.
Figure 9
| Atom (in [N(CH3)4]+) | Charge (C) | Atom (in [P(CH3)4]+) | Charge (C) |
| N | -0.295 | P | 1.666 |
| C | -0.483 | C | -1.060 |
| H | 0.269 | H | 0.298 |
The lower the value in the table above, the more negatively charged the atom, i.e. the more electron density around it. The nitrogen is has a more negative charge than the phosphorus as it is more electronegative so has more electron density around it. Nitrogen has a high Pauling electronegativity of 3.04, whereas phosphorus is 2.19[1]. As a result of its lone pair being tightly held, less electron density is donated to form the dative bond with carbon so the carbon has less electron density around it, as reflected in the table above. Nitrogen is more electronegative than phosphorus as it has a smaller atomic radius so the effective nuclear charge felt by the electrons is increased. There is also less shielding by inner electrons for nitrogen as it has fewer shells of electrons.
References
- ↑ 1.0 1.1 Pauling L. The Nature of the Chemical Bond. IV. The Energy of Single Bonds and the Relative Electronegativity of Atoms. Journal of the American Chemical Society. 1932:54(9):3570-3582. doi: 10.1021/ja01348a011 [Date Accessed: 16 May 2019]
- ↑ Cottrell T L. The Strengths of Chemical Bonds. 1958. 2. Available from: http://www.wiredchemist.com/chemistry/data/bond_energies_lengths.html [Date Accessed: 19 May 2019]