Rep:Mod:ialrirpl
Module 3
Part 1: The Cope Rearrangement
Isomers of 1,5-hexadiene
Several different isomers of 1,5-hexadiene were optimized using HF/3-21G theory, and the results compared.
| Anti1 | Anti2 | Anti4 | Gauche2 | Gauche3 | |
|---|---|---|---|---|---|
| Gaussview |  |
 |
 |
 |
|
| Point Group | C2 | Ci | C1 | C2 | C1 |
| Energy (Hartrees) | -231.69260235 | -231.69253528 | -231.69097055 | -231.69166701 | -231.69266120 |
| Relative Energy (Hartrees) | 0.00005885 | 0.00012592 | 0.00169065 | 0.00099419 | 0.00000000 |
| Relative Energy (kJ/mol) | 0.155 | 0.331 | 4.44 | 2.61 | 0.00 |
| Log file | File:Ialrirpl anti1.log | File:Ialrirpl anti2.log | File:Ialrirpl anti4.log | File:Ialrirpl gauche2.log | File:Ialrirpl gauche3.log |
The anti2 (Ci) isomer was then re-optimised using DFT/6-31G. The geometries at each level of theory are shown below:
| HF/3-21G | DFT/6-31Gd |
|---|---|
 |
|
As we can see, there is no significant difference in geometry.
The vibrations of the above optimized structure were then calculated, to ensure that the geometry shown was a minimum. The log files for the optimization and the vibration calculations are below, and the calculated IR spectrum is inset.

Optimization:File:Ialrirpl anti2 631g.log
Vibration:File:Ialrirpl anti2 631g vib.log
| Energy (Hartrees) | |
|---|---|
| Electronic + Zero Point Energies | -234.469212 |
| Electronic + Thermal Energies | -234.461856 |
| Electronic + Thermal Enthalpies | -234.460912 |
| Electronic + Thermal Free Energies | -234.500821 |
The Chair and Boat Transition States
The chair and boat transition structures for the cope rearrangement were calculated. First an allyl fragment was optimized using HF/3-21G theory. This was then used to make an approximation of both the chair and the boat transition states. Each of these was optimized to a TS(Berny) with the same level of theory, and the imaginary frequencies observed. In each case, the calculated imaginary frequency corresponded to the cope rearrangement. An IRC calculation was then run using each of these. The maximum and the minimum points were taken, and these were then optimized using DFT/6-31Gd theory.
The log file from the allyl optimisation is available here: File:Ialrirpl allyl.log
| Chair | Boat | |
|---|---|---|
| Gaussview TS |  |
|
| TS Opt+Freq Log File | File:Ialrirpl chair optfreq.log | File:Ialrirpl boat optfreq.log |
| Imaginary Frequency gif |  |

|
| IRC Image | 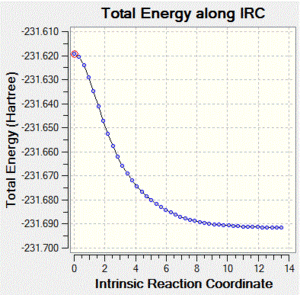 |
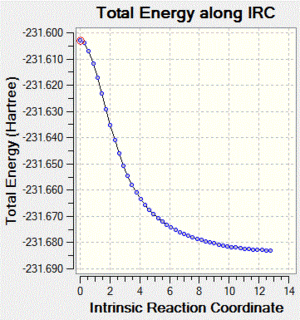
|
| IRC Log File | File:Ialrirpl chair irc.log | File:Ialrirpl boat irc.log |
| Final Optimized Structure | 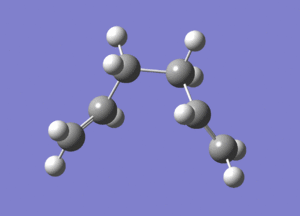 |
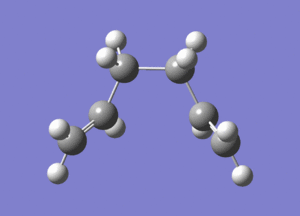
|
| Final Structure Log File | File:Ialrirpl chair final.log | File:Ialrirpl boat final.log |
| Final Optimized TS Log File | File:Ialrirpl chair ts 631.log | File:Ialrirpl boat ts 631.log |
The chair transition state yields isomer gauche2, and the boat transition state yields isomer gauche3.
| Chair at HF/3-21G | Chair at DFT/6-31G* | Boat at HF/3-21G | Boat at DFT/6-31G* | |
|---|---|---|---|---|
| Energy starting material (Hartrees) | -231.69266120 | -234.61132934 | -231.69266120 | -234.61132934 |
| Energy of TS (Hartrees) | -231.61932097 | -234.55693192 | -231.60280247 | -234.54309303 |
| Activation Energy (Hartrees) | 0.07334023 | 0.05439742 | 0.08985873 | 0.06823631 |
| Activation Energy (kJ/mol) | 192.55 | 142.82 | 235.92 | 179.15 |
These do correspond well with experimental values.
Part 2: The Diels Alder Reaction
Ethene and cis-butadiene
The structures of ethene and cis-butadiene were optimized using the HF/3-21G level of theory. These structures were used to construct a transition state, which was optimized using the same level of theory to a TS (Berny). The imaginary frequency observed (right) corresponded to the Diels-Alder cycloaddition. The IRC was plotted (below), and gave the expected Diels-Alder adduct as the product. This was then optimized using the same theory level as above.

| Files above | IRC path |
|---|---|
| Optimized ethene log file:File:Ialrirpl ethene.log Optimized cis-butadiene log file:File:Ialrirpl cisbutadiene.log |
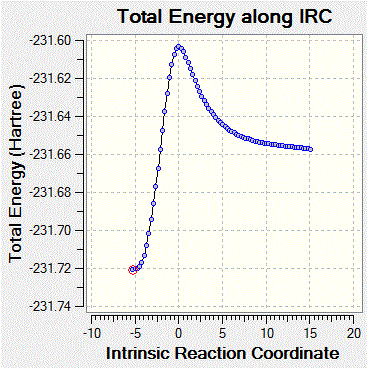
|
The orbitals of ethene, cis-butadiene, the transition state and the adduct were plotted, and the symmetries compared.
| Ethene | Cis-butadiene | Transition State | Diels-Alder adduct | |
|---|---|---|---|---|
| Gaussview |  |
 |
 |
|
| HOMO | 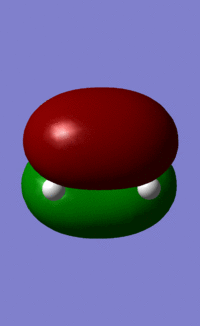 |
 |
 |
|
| Symmetry | s | a | s | s |
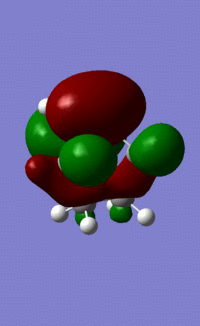
| LUMO | 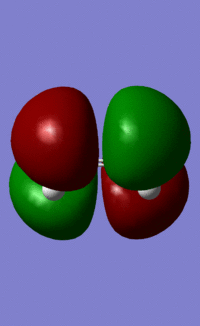 |
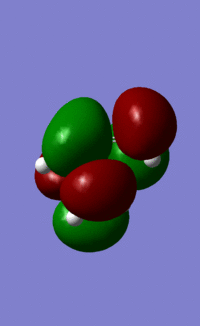 |
 |

|
| Symmetry | a | s | s | a |
The HOMO of ethene and the LUMO of cisbutadiene are the orbitals that combine to give the transition state orbitals
Diels-Alder and regioselectivity
The Diels-Alder cycloaddition between 1,3-cyclohexadiene and maleic anhydride gives two possible products: the endo (where the C-O-C is bent /towards/ the double bond) and the exo (where the C-O-C is bent /away/ from the double bond) isomers.
| Exo | Endo | |
|---|---|---|
| Chemdraw | 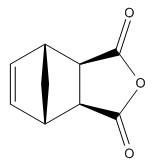 |

|
| Gaussview |  |

|
| Relative energy (Hartrees) | 0.00250473 | 0.0000000 |
| Relative energy (kJ/mol) | 6.58 | 0.00 |
| Log file | File:Ialrirpl exo631g.log | File:Ialrirpl endo631g.log |
The endo product is lower in energy than the exo product, meaning that we would expect it to predominate. This tallies well with experimental evidence, but as the reaction is under kinetic control, the endo transition state must also be lower in energy.
The exo and endo transition states were modelled.
| Exo | Endo | |
|---|---|---|
| Gaussview |  |

|
| Forming C-C bond distance (Å) | 2.268 | 2.291 |
| C-O-C to cyclohexa-1,2-diene distance (Å) | 3.028 | 2.990 |
| Energy (Hartrees) | -612.67931091 | -612.68339670 |
| Relative energy (Hartrees) | 0.00408579 | 0.00000000 |
| Relative energy (kJ/mol) | 10.73 | 0.00 |
| Log file | File:Ialrirplexo TS 631g.log | File:Ialrirplendo TS 631g.log |
The endo transition state is lower in energy than the exo transition state, which corresponds with experiment. The exo form is more strained despite the C-O-C fragment being further away from the corresponding cyclohexadiene carbons. This at first seems counterintuitive. The endo form is favoured due to the secondary orbital overlap effect. This occurs when a bonding and an anti-bonding orbital overlap and interact, mixing to form new orbitals, resulting in stabilisation of both molecules, or in this case, of one conformation of a transition over another.
The HOMO of the exo and endo isomers, and the transition states:
| Exo | Endo | |
|---|---|---|
| Orbitals | 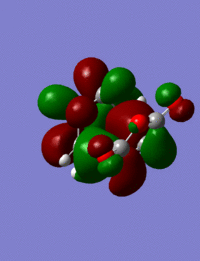 |

|
| Symmetry | s | s |
Both isomers have very similar HOMOs, with nodes along the C-O bonds of the maleic anhydride region. The ether oxygen has larger orbitals in the endo than the exo, suggesting it contributes more to this orbital. This fits well with theory, as the endo isomer would be expected to have a lower energy HOMO, and electronegative atoms contribute more to lower energy orbitals.
