Rep:Mod:hyt215 IMM2
NH3 molecule
Information
|
Click here to view log file. | |||
| Calculation Details | |||
|---|---|---|---|
| Calculation Method | RB3LYP | ||
| Basis Set | 6-31G(d,p) | ||
| Point Group | C3v | ||
| Calculation Results | |||
| Final Energy E(RB3LYP)/a.u. | -56.55776873 | ||
| RMS Gradient/a.u. | 0.00000485 | ||
| Bond Length/Å | 1.01798 | ||
| Bond Angle/° | 37.129 | ||
"Item" Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000070 0.001800 YES RMS Displacement 0.000033 0.001200 YES
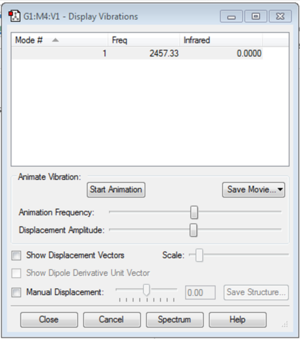
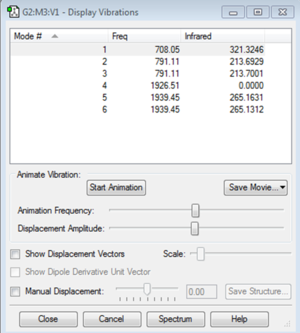
Vibration Modes
The image above shows the screenshot of vibration modes of NH3. As there are no negative frequencies, the energy of the molecule is at its local minima.
| Number of Modes (based on 3N-6 rule) | 6 |
|---|---|
| Degenerate Modes | 2 and 3, 5 and 6 |
| "Bending" Vibrations | 1089.54 Hz (1), 1693.95 Hz (2 and 3) |
| "Bond Stretch" Vibrations | 3461.29 Hz (4), 3589.82 Hz (5 and 6) |
| Highly Symmetric Mode | 4 |
| "Umbrella" Mode | 1 |
| Bands Expected in an Experimental Spectrum | 4 |
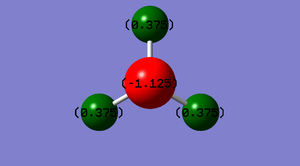
Atomic Charge
| Atom | Charge |
|---|---|
| Hydrogen | +0.375 |
| Nitrogen | -1.125 |
Being more electronegative than hydrogen atoms, the nitrogen atom is more electron withdrawing and therefore carries a negative charge. Being less electronegative, the hydrogen atoms carry a positive charge.
N2 molecule
Information
|
Click here to view log file. | |||
| Calculation Details | |||
|---|---|---|---|
| Calculation Method | RB3LYP | ||
| Basis Set | 6-31G(d,p) | ||
| Point Group | D∞h | ||
| Calculation Results | |||
| Final Energy E(RB3LYP)/a.u. | -109.52412868 | ||
| RMS Gradient/a.u. | 0.00000060 | ||
| Bond Length/Å | 1.10550 | ||
"Item" Table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
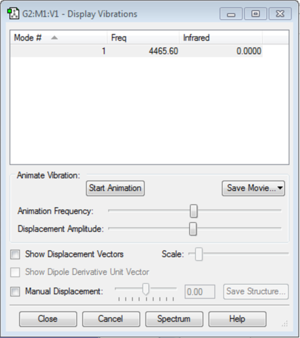
Vibration Modes
The image above shows the screenshot of vibration modes of N2. As there are no negative frequencies, the energy of the molecule is at its local minima. Due to the absence of a net dipole moment, there were no IR active vibrational modes present.
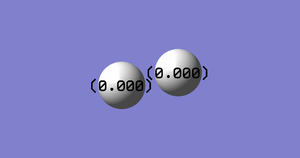
Atomic Charge
| Atom | Charge |
|---|---|
| Nitrogen | 0.000 |
As the electronegativity of both atoms are the same, the charge is 0.000.
H2 molecule
Information
|
Click here to view log file. | |||
| Calculation Details | |||
|---|---|---|---|
| Calculation Method | RB3LYP | ||
| Basis Set | 6-31G(d,p) | ||
| Point Group | D∞h | ||
| Calculation Results | |||
| Final Energy E(RB3LYP)/a.u. | -1.17853936 | ||
| RMS Gradient/a.u. | 0.00000222 | ||
| Bond Length/Å | 0.74280 | ||
"Item" Table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000005 0.001800 YES RMS Displacement 0.000007 0.001200 YES
Vibration Modes
The image above shows the screenshot of vibration modes of H2. As there are no negative frequencies, the energy of the molecule is at its local minima. Due to the absence of a net dipole moment, there were no IR active vibrational modes present.
Atomic Charge
| Atom | Charge |
|---|---|
| Hydrogen | 0.000 |
As the electronegativity of both atoms are the same, the charge is 0.000.
Energetics of Haber-Bosch process
N2 + 3H2 → 2NH3
The table below shows the energy of the above reaction in atomic units (a.u.).
| E(NH3) | -56.55776873 |
|---|---|
| 2*E(NH3) | -113.11553746 |
| E(N2) | -109.52412868 |
| E(H2) | -1.17853936 |
| 3*E(H2) | -3.53561808 |
| ΔE=2*E(NH3) - [E(N2) + 3*E(H2)] | -0.05579070 |
The energy change is ΔE= -0.05579070×2625.5= -146.48 kJmol-1. The negative sign of enthalpy change indicates that the reaction is exothermic and energy is released into the surroundings. The decrease in energy indicates that ammonia is more stable than the gaseous reactants.
[AlCl4]- molecule
Information
|
Click here to view log file. | |||
| Calculation Details | |||
|---|---|---|---|
| Calculation Method | RB3LYP | ||
| Basis Set | 6-31G(d,p) | ||
| Point Group | Td | ||
| Calculation Results | |||
| Final Energy E(RB3LYP)/a.u. | -2083.61641374 | ||
| RMS Gradient/a.u. | 0.00000606 | ||
| Bond Length/Å | 2.17690 | ||
| Bond Angle/° | 109.471 | ||
"Item" Table
Item Value Threshold Converged? Maximum Force 0.000012 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000080 0.001800 YES RMS Displacement 0.000043 0.001200 YES
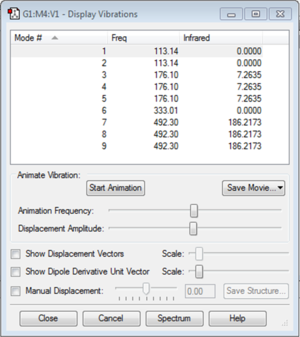
Vibration Modes
The image above shows the screenshot of vibration modes of [AlCl4]-. As there are no negative frequencies, the energy of the molecule is at its local minima.
The table below shows the vibrations of the molecule. As [AlCl4]- is non-linear, there are 3(5)-6=9 vibrational modes, given by the equation 3N-6 where N is the number of atoms.
The image above shows the predicted IR spectra for [AlCl4]-. As there are two energy levels at which [AlCl4]- are IR active, there are two peaks in the spectrum at 176.10 cm-1 and 492.30 cm-1.
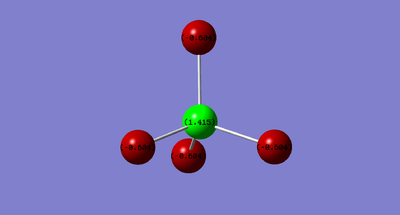
Atomic Charge
| Atom | Charge |
|---|---|
| Aluminium | +1.415 |
| Chlorine | -0.604 |
Being more electronegative than aluminium, chlorine atoms are more electron withdrawing and therefore carries a negative charge. Being less electronegative, the aluminium atoms carry a positive charge.
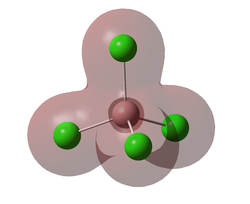
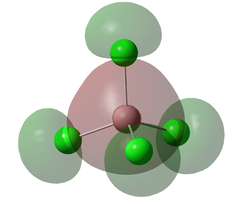
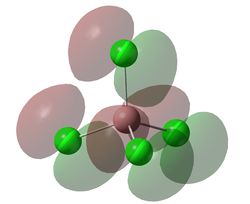
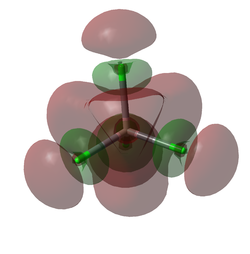
Molecular Orbitals
A molecular orbital diagram was calculated using GaussView with a degeneracy threshold of 0.00001. A small degeneracy threshold was chosen to allow for small differences in energy to be visibly shown.
The table below describes some of the MOs shown above.
AlCl3 molecule
Information
|
Click here to view log file. | |||
| Calculation Details | |||
|---|---|---|---|
| Calculation Method | RB3LYP | ||
| Basis Set | 6-31G(d,p) | ||
| Point Group | D3h | ||
| Calculation Results | |||
| Final Energy E(RB3LYP)/a.u. | -244.20693747 | ||
| RMS Gradient/a.u. | 0.00005838 | ||
| Bond Length/Å | 1.58678 | ||
| Bond Angle/° | 120 | ||
"Item" Table
Item Value Threshold Converged? Maximum Force 0.000117 0.000450 YES RMS Force 0.000076 0.000300 YES Maximum Displacement 0.000825 0.001800 YES RMS Displacement 0.000540 0.001200 YES
Vibration Modes
The image above shows the screenshot of vibration modes of AlCl3. As there are no negative frequencies, the energy of the molecule is at its local minima.
Atomic Charge
| Atom | Charge |
|---|---|
| Aluminium | +1.135 |
| Chlorine | -0.378 |
Being more electronegative than aluminium, chlorine atoms are more electron withdrawing and therefore carries a negative charge. Being less electronegative, the aluminium atoms carry a positive charge.