Rep:Mod:hys116 yr2
EX3 section
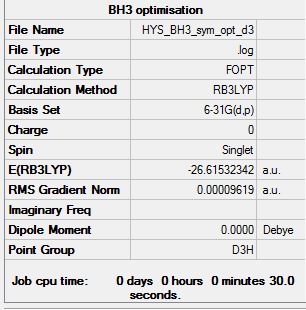
BH3
B3LYP/6-21G (d,p)
Item Value Threshold Converged? Maximum Force 0.000192 0.000450 YES RMS Force 0.000126 0.000300 YES Maximum Displacement 0.000763 0.001800 YES RMS Displacement 0.000500 0.001200 YES
Low frequencies --- -0.2260 -0.1036 -0.0055 48.0278 49.0875 49.0880 Low frequencies --- 1163.7224 1213.6715 1213.6741
optimised BH molecule |
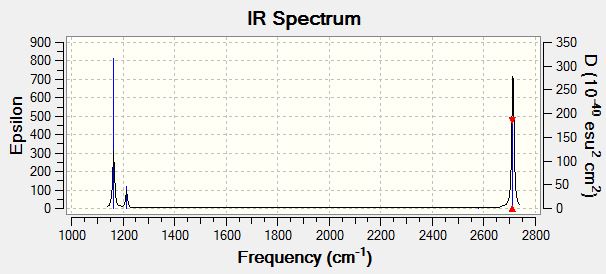
IR
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1164 | 92 | A2" | yes | out-of-plane bend |
| 1214 | 14 | E' | slight | bend |
| 1214 | 14 | E' | slight | bend |
| 2580 | 0 | A1' | no | symmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
Although there are 6 vibrations in the table, there are not 6 vibrations seen in the IR spectrum. There are two pairs of degenerate vibrations so these are only seen once and some vibrations are not IR active. For a molecule to be IR active, there must be a change in dipole moment. The A1 symmetric stretch does not involve an overall change in dipole moment so is not IR active so is not seen in the spectrum. Vibrations with a larger change in dipole moment will show a more intense signal e.g. E' asymmetric stretch.
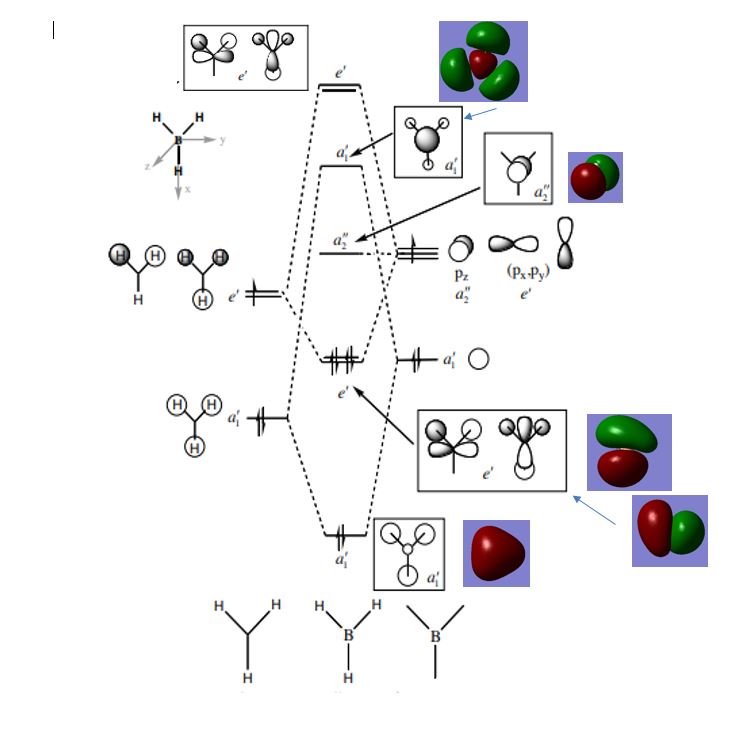
MO diagram
Below is the MO diagram for BH3 showing the LCAO as well as the corresponding computed MOs.
(Figure 3 MO diagram for BH3, Lecture 4 Tutorial Problem Model Answers, P. Hunt, http://www.huntresearchgroup.org.uk/teaching, accessed 04/05/18)
The LCAO MOs are very similar to the computed MOs with the same regions of bonding and antibonding electron density seen in both representations. This suggests that qualitative MO theory is a very useful technique for approximating the shapes and distributions of molecular orbitals. This qualitative theory also allows an accurate energy ordering of the MOs to be created.
Ng611 (talk) 17:54, 17 May 2018 (BST) Well analyzed but missing a few higher energy antibonding orbitals. From comparing the calculated and qualitative MOs, are there any differences at all between them at all?
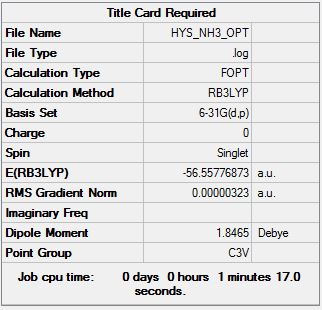
NH3
B3LYP/6-21G (d,p)
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Low frequencies --- -8.5646 -8.5588 -0.0044 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
optimised NH molecule |
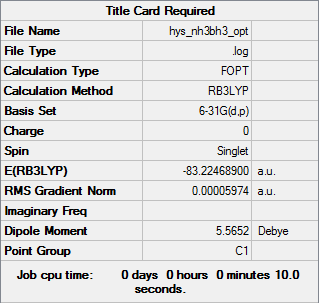
NH3BH3
B3LYP/6-21G (d,p)
Item Value Threshold Converged? Maximum Force 0.000123 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000541 0.001800 YES RMS Displacement 0.000296 0.001200 YES
Low frequencies --- -0.0011 -0.0010 0.0006 10.2334 22.3402 34.7768 Low frequencies --- 265.3599 632.2648 638.9861
optimised NHBH molecule |
Energy Calculations
The association energy for the reaction of NH3 + BH3 to give NH3BH3 is shown below:
E(NH3)= -56.55777 a.u.
E(BH3)= -26.61532 a.u.
E(NH3BH3)= -83.22469 a.u.
Association energy, ΔE = E(NH3BH3) - [E(NH3)+E(BH3)] = -0.05160 a.u
= -135 kJ/mol
This energy calculation shows that the B-N dative bond is a weak bond. The bond enthalpy, or the dissociation energy, is +135 kj/mol, which is much lower than most organic bonds, for example C-C ~350 kj/mol.
Ng611 (talk) 21:23, 15 May 2018 (BST) Remember to cite your bond values (ideally from a textbook, databook, or paper)!
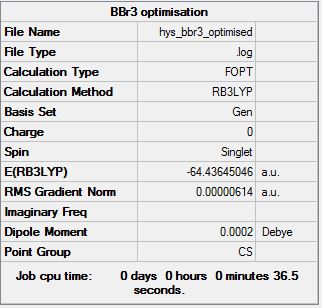
BBr3
B3LYP
Basis set = 6-31G (d,p) for B, LanL2DZ (pseudo-potential) for Br
Item Value Threshold Converged?
Maximum Force 0.000010 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000045 0.001800 YES
RMS Displacement 0.000032 0.001200 YES
http://hdl.handle.net/10042/202315
Low frequencies --- -1.9018 -0.0002 -0.0001 -0.0001 1.5796 3.2831 Low frequencies --- 155.9053 155.9625 267.7047
optimised NHBH molecule |
Project Section
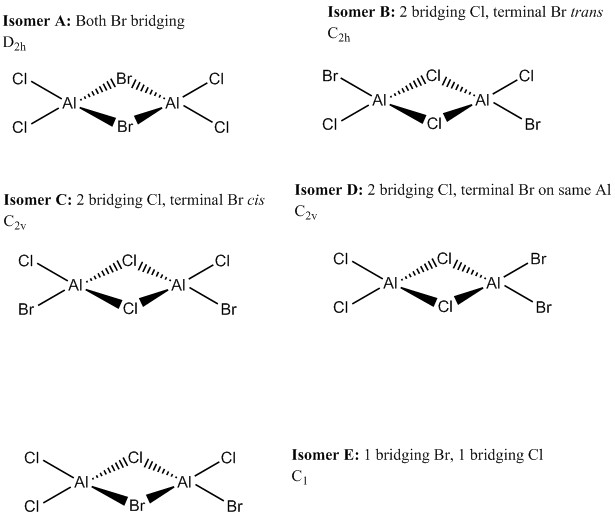
Al2Cl4Br2 exists as dimers, with 2 bridging atoms, in structures analogous to B2H6, with 2 bridging atoms. There are 5 isomers of Al2Cl4Br2 with different bridging atoms and different arrangements of the terminal atoms. These isomers are shown in the diagrams below, where the point group of each isomer was also assigned based on their symmetry:
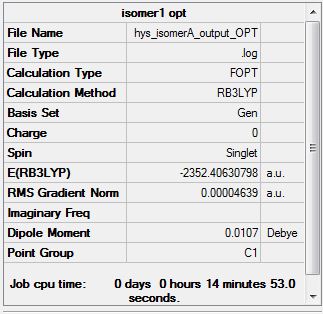
Isomer A
Isomer A has both Br atoms in the bridging position as shown in the previous diagram. Its energy was computed and is shown below:
B3LYP
Basis set = 6-31G (d,p) for Al, Cl, LanL2DZ (pseudo-potential) for Br
Item Value Threshold Converged? Maximum Force 0.000089 0.000450 YES RMS Force 0.000033 0.000300 YES Maximum Displacement 0.001000 0.001800 YES RMS Displacement 0.000446 0.001200 YES
Media:hys_isomerA_output_OPT.log
optimised AlClBr molecule |
Isomer B
Isomer B has 2 Cl ions in the bridging positions and the 2 terminal Br ions arranged trans to each other. Its energy was computed and is shown below:
B3LYP
Basis set = 6-31G (d,p) for Al, Cl, LanL2DZ (pseudo-potential) for Br
Item Value Threshold Converged? Maximum Force 0.000033 0.000450 YES RMS Force 0.000014 0.000300 YES Maximum Displacement 0.000464 0.001800 YES RMS Displacement 0.000235 0.001200 YES
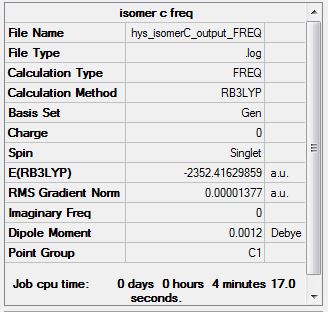
Media:hys_isomerC_output_FREQ.log
Low frequencies --- -4.9393 -0.0022 0.0013 0.0016 1.5295 1.9989
Low frequencies --- 18.1817 49.1295 73.0124
optimised AlClBr molecule |
Relative Energies
Using the methods and basis sets described previously, the energy of isomer A was found to be -2352.40630 a.u. and isomer B: -2352.41630 a.u.
A relative energy calculation was carried out and isomer B, with two bridging Cl atoms and the two Br atoms trans to each other, was found to be more stable than isomer A, with two bridging Br atoms by 26 kj/mol.
B is more stable, suggesting bridging Cl ions are more stable than bridging Br ions. This is because Br is a much bigger atom, so when two Br atoms are in bridging positions there is large steric hinderance. Br valence orbitals are also more diffuse than those of Cl.
Dissociation
The Al2Cl4Br2 dimer dissociates into the monomer AlCl2Br and the dissociation for this reaction was calculated below:
Low energy isomer B is used for calculations.
E(Al2Cl4Br2) = -2352.41630 a.u.
E(AlCl2Br) = -1176.19014 a.u.
Dissociation energy, ΔE= (2 x E(AlCl2Br)) - E(Al2Cl4Br2) = + 0.03602 a.u.
= + 95 kJ/mol
The dimer is more stable than the isolated monomers, as the electron deficiency of Al is relieved by the formation of bridging bonds.
MOs
A MO calculation was carried out for isomer B, the lower energy isomer. All the occupied valence MOs (NUMBER) and the lowest 5 unoccupied MOs were visualised. A selection are shown and analysed below, along with their LCAO representation:
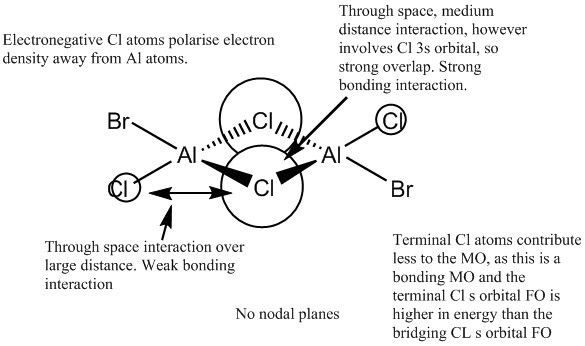
MO 31
Overall this MO is bonding
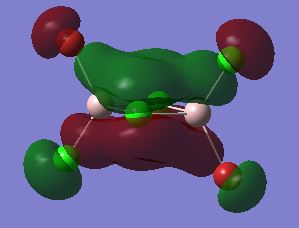
MO 40
Overall this MO is strongly antibonding.
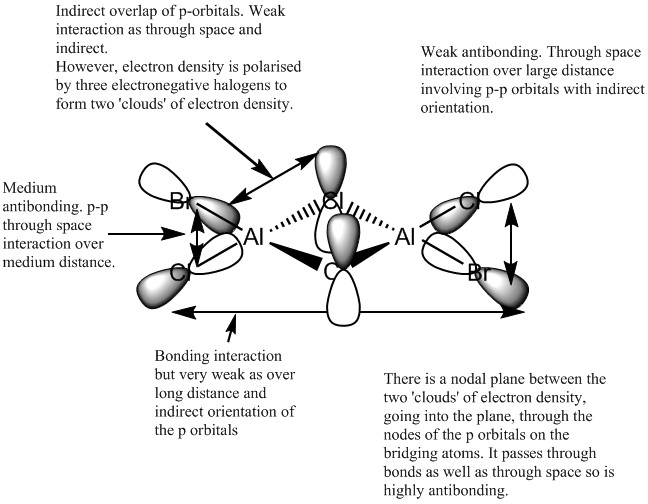
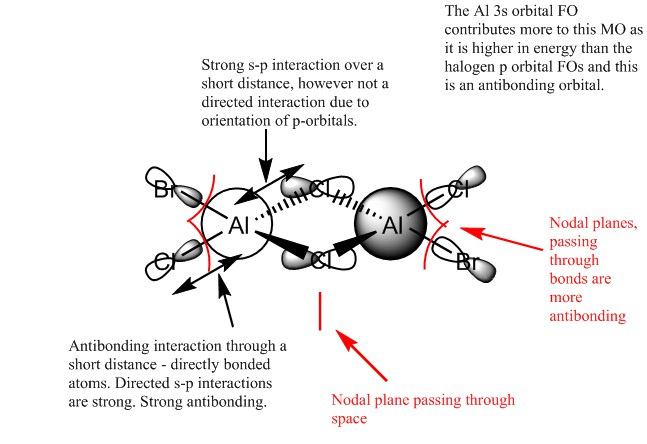
MO 56
Overall this MO is very strongly antibonding
Ng611 (talk) 21:28, 15 May 2018 (BST)Very good report. A couple of items were missing (MOs for 2e' and low mode freq analysis for isomer A) but otherwise thorough and well written.