Rep:Mod:hys116
NH3
Summary
Molecule name = Ammonia, NH3
Calculation method = RB3LYP
Basis Set = 6-31G(d,p)
Final energy, E(RB3LYP) = -56.55776873 a.u
Point group = C3V
N-H bond distance = 1.01798 Å
H-N-H bond angle = 105.74115°
The results obtained for bond length and angle are very close to the accepted values of 1.012 Å and 106.7° [1] showing that the results obtained from Gaussian are accurate.
NH3 |
Item table
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Vibrations
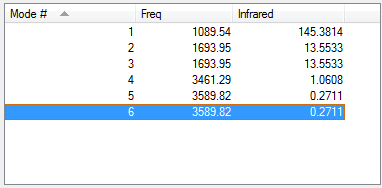
From 3N-6 rule, ammonia is expected to have 3(4)-6 = 6 vibrational modes. Modes 2 and 3 are degenerate and modes 5 and 6 are also degenerate. Modes 1,2 and 3 are bends, 4,5 and 6 are stretches. Mode 4 is highly symmetric and mode 1 is known as the 'umbrella' mode. 2 bands would be expected in the experimental spectrum of ammonia.
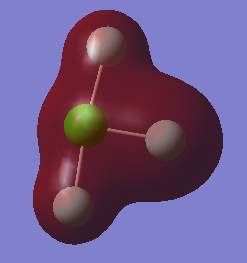
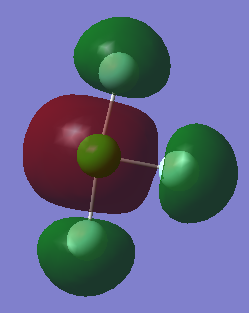
Charges
The charge on the nitrogen molecule is -1.125 and the charge on each hydrogen is +0.375.This is as expected because nitrogen is more electronegative than fluorine so withdraws electrons and has a more negative charge.
Link to optimisation
The optimisation file is liked to here
N2
Summary
Molecule name = Nitrogen, N2
Calculation method = RB3LYP
Basis Set = 6-31G(d,p)
Final energy, E(RB3LYP) = -109.52412868 a.u
Point group = D∞h
N-N bond distance = 1.10550 Å
The N-N bond length obtained is close to the accepted value of 1.158 Å [2]
suggesting that the calculation is correct and the results obtained accurate.
N2 |
Item table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Vibrations
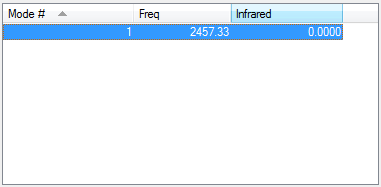
There are no negative frequencies and no bands would be expected in the infrared spectrum as there is no change in dipole moment as nitrogen is homodinuclear.
Link to optimisation
The optimisation file is liked to here
H2
Summary
Molecule name = Hydrogen, H2
Calculation method = RB3LYP
Basis Set = 6-31G(d,p)
Final energy, E(RB3LYP) = -1.17853936 a.u
Point group = D∞h
H-H bond distance = 0.74279 Å
The bond length obtained is close to the expected value of 0.74 Å [3] suggesting that the calculated bond length is accurate.
H2 |
Item table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Vibrations
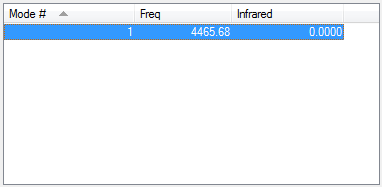
There are no negative frequencies and no expected bands in the experimental spectrum as there is no change in dipole moment as hydrogen is homodinuclear.
Link to optimisation
The optimisation file is liked to here
Haber-Bosch Process energy
E(NH3)= -56.55776873 a.u
2*E(NH3)= -113.1155375 a.u
E(N2)= -109.52412868 a.u
E(H2)= -1.17853936 a.u
3*E(H2)= -3.53561808 a.u
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u
= -146.4784829 kJ/mol
~ -146.48 kJ/mol
ΔE is negative therefore the reaction is exothermic and the ammonia product is more thermodynamically stable than the reactants.
Project molecule: ClF3
Summary
Molecule name = Chlorine trifluoride, ClF3
Calculation method = RB3LYP
Basis Set = 6-31G(d,p)
Final energy, E(RB3LYP) = -759.46531688 a.u
Point group = C2V
Cl-F bond distance = 1.72863 Å, 1.65143 Å
F-Cl-F bond angle = 87.14037°,174.28073°
Following VSEPR theory, ClF3 forms a T-shaped molecule as there are 2 lone pairs and 3 bonding pairs around the central atom. This results in 2 longer Cl-F bonds between the chlorine and the axial fluorines and one shorter bond with the equatorial fluorine. The bond angles are slightly less than 90° and 180° due to the lone pair repulsions.
ClF3 |
Item table
Item Value Threshold Converged?
Maximum Force 0.000050 0.000450 YES
RMS Force 0.000028 0.000300 YES
Maximum Displacement 0.000204 0.001800 YES
RMS Displacement 0.000134 0.001200 YES
Vibrations
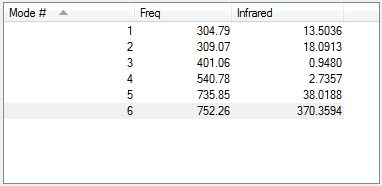
From 3N-6 rule, ClF3 is expected to have 3(4)-6 = 6 vibrational modes. Modes 1 and 2 are degenerate. Modes 1,2 and 3 are bends, 4,5 and 6 are stretches. Modes 1, 4 and 5 are highly symmetric. 3 bands would be expected in the experimental spectrum of ClF3, from modes 1/2, 5 and 6.
Charges
The charge on the chlorine molecule is +1.225 and the charge on each fluorine is -0.454.This is as expected because fluorine is more electronegative than chlorine.
Molecular Orbitals
The energies of the molecular orbitals are shown here:

Molecular orbital 5, with energy -9.68 a.u is shown below:
The MO is made of the 2s bonding AO on Cl. It is a bonding MO. It is deep in energy as it is formed from the core AO on chlorine, not the valence AO and there is no overlap with other MOs, therefore this MO is not involved in bonding.
Molecular orbital 8, with energy -7.45 a.u is shown below:
This is the bonding MO made from the 2p AO on chlorine. It is perpendicular to the bonds so is a pi MO. MOs 6 and 7 have the same energy as 8; they are degenerate. This is beacuse they are formed from the 3 2p AOs in different planes (x,y,z). The MOs overlap; they are occupied and involved in bonding.
Molecular orbital 9, with energy -1.28 a.u is shown below:
This is a combination of the valence s AOs: the 3s on Cl and 23 on F. This is the bonding MO, where the bonding is so extensive that only one surface is seen. This explains why it is significantly higher in energy than the previous MOS.
Molecular orbital 11, with energy -1.17 a.u is shown below:
This is an antibonding orbital formed from the 2p AOs on F. There is no overlap between orbitals so these MOs are not involved in bonding.
Molecular orbital 12, with energy -0.89 a.u is shown below:
This is an antibonding MO formed from the 3p AO on Cl and the 2p AOs on F. There is no overlap so this MO is not involved in bonding
Link to Optimisation
The optimisation file is liked to here
References
- ↑ CRC Handbook of Chemistry and Physics, 94th ed. http://www.hbcpnetbase.com. Page 9-26. Retrieved 7 March 2017.
- ↑ F. Liang et al. (2016), Nature Scientific Reports 6, 31559 http://www.nature.com/articles/srep31559#t1 Retrieved 7 March 2017.
- ↑ Bond Lengths and Energies http://www.science.uwaterloo.ca/~cchieh/cact/c120/bondel.html Retrieved 7 March 2017.