Rep:Mod:ht309mod3
3rd Year Computational Labs: Physical
The Cope Rearrangement Tutorial
Introduction

In this investigation the low-energy minima and the transition structures of 1,5-hexadiene were located to determine the preferred reaction mechanism for the Cope Rearrangement, i.e. [3,3]-sigmatropic shift rearrangement using Gaussian. Literature[1] reports the mechanism to proceed via an aromatic loose-chair transition state, i.e. a concerted transition state.
Optimisation of 1,5-Hexadiene
At first, several conformers of 1,5-hexadiene were assessed on GaussView using the HF/3-21g method and basis set. These conformers readily interconvert via free rotation of C-C σ bonds at room temperature. As well as optimising the conformers, the energy of the conformers were determined.
| Conformer | Structure | Point Group | Energy (HF/3-21G)/Eh | Energy (DFT/B3LYP/6-)/Eh | Σ(Eelec + ZPE)/Eh | Σ(Eelec + Etherm)/Eh | Σ(Helec + Htherm)/Eh | Σ(Gelec + Gtherm)/Eh | DOI (freq.log) |
|---|---|---|---|---|---|---|---|---|---|
| Anti 1 | C2 | -231.69260 | -234.61180 | -234.46929 | -234.46197 | -234.46102 | -234.50016 | DOI:10042/to-10028 | |
| Anti 2 | Ci | -231.69254 | -234.61170 | -234.46921 | -234.46186 | -234.46091 | -234.50082 | DOI:10042/to-10024 | |
| Anti 3 | C2h | -231.68907 | -234.60964 | -234.46699 | -234.45982 | -234.45887 | -234.49734 | DOI:10042/to-10029 | |
| Anti 4 | C1 | -231.69097 | -234.61079 | -234.46819 | -234.46094 | -234.46000 | -234.49948 | DOI:10042/to-10027 | |
| Gauche 1 | C2 | -231.68772 | -234.60788 | -234.46525 | -234.45812 | -234.45718 | -234.49548 | DOI:10042/to-10025 | |
| Gauche 2 | C2 | -231.69167 | -234.61070 | -234.46821 | -234.46094 | -234.45600 | -234.49910 | DOI:10042/to-10070 | |
| Gauche 3 | C1 | -231.69266 | -234.61133 | -234.46849 | -234.46146 | -234.46052 | -234.50011 | DOI:10042/to-10030 | |
| Gauche 4 | C2 | -231.69153 | -234.61048 | -234.46778 | -234.46052 | -234.45958 | -234.49869 | DOI:10042/to-10031 | |
| Gauche 5 | C1 | -231.68962 | -234.60911 | -234.46637 | -234.45921 | -234.45827 | -234.49744 | DOI:10042/to-10057 | |
| Gauche 6 | C1 | -231.68916 | -234.60889 | -234.46628 | -234.45910 | -234.45816 | -234.49737 | DOI:10042/to-10032 |
| Conformation | DOI |
|---|---|
| Anti 1 | HF/3-21GDOI:10042/to-10077 DFT/B3LYP/6-31G*DOI:10042/to-10026 |
| Anti 2 | HF/3-21GDOI:10042/to-10072 DFT/B3LYP/6-31G*DOI:10042/to-10075 |
| Anti 3 | HF/3-21GDOI:10042/to-10080 DFT/B3LYP/6-31G*DOI:10042/to-10071 |
| Anti 4 | HF/3-21GDOI:10042/to-10078 DFT/B3LYP/6-31G*DOI:10042/to-10079 |
| Gauche 1 | HF/3-21GDOI:10042/to-10074 DFT/B3LYP/6-31G*DOI:10042/to-10073 |
| Gauche 2 | HF/3-21GDOI:10042/to-10090 DFT/B3LYP/6-31G*DOI:10042/to-10091 |
| Gauche 3 | HF/3-21GDOI:10042/to-10082 DFT/B3LYP/6-31G*DOI:10042/to-10083 |
| Gauche 4 | HF/3-21GDOI:10042/to-10086 DFT/B3LYP/6-31G*DOI:10042/to-10084 |
| Gauche 5 | HF/3-21GDOI:10042/to-10088 DFT/B3LYP/6-31G*DOI:10042/to-10089 |
| Gauche 6 | HF/3-21GDOI:10042/to-10084 DFT/B3LYP/6-31G*DOI:10042/to-10085 |
The HF/3-21g method is primitive, thus the conformers were reoptimised using the DFT B3LYP method with 6-21G* basis set.
| Conformation | Energy[HF/3-21G]/Eh | (Rel)ΔE/kcalmol-1 | Energy[DFT/B3LYP/6-31G]/Eh | (Rel)ΔE/kcalmol-1 |
|---|---|---|---|---|
| Anti 1 | -231.69260 | 0.037 | -234.61180 | 0.000 |
| Anti 2 | -231.69254 | 0.079 | -234.61170 | 0.063 |
| Anti 3 | -231.68907 | 2.253 | -234.60964 | 1.355 |
| Anti 4 | -231.69097 | 1.061 | -234.61079 | 0.634 |
| Gauche 1 | -231.68772 | 3.103 | -234.60788 | 2.460 |
| Gauche 2 | -231.69167 | 0.624 | -234.61070 | 0.690 |
| Gauche 3 | -231.69266 | 0.000 | -234.61133 | 0.295 |
| Gauche 4 | -231.69153 | 0.710 | -234.61048 | 0.828 |
| Gauche 5 | -231.68962 | 1.911 | -234.60911 | 1.688 |
| Gauche 6 | -231.68916 | 2.197 | -234.60889 | 1.826 |
The results were surprising in that the HF method predicted the Gauche 3, Anti 1, and Anti 2 forms to be most stable whereas the DFT B3LYP method predicted only predicted the Anti 1 and Anti 2 formers to be most stable.
Key Notes:
Σ(Eelec + ZPE)/Eh - refers to the potential energy at 0 K including the zero-point vibrational energy.
Σ(Eelec + Etherm)/Eh - refers to the energy at 298.15 K and 1 atm of pressure which includes contributions from the translational, rotational, and vibrational energy modes at this temperature.
Σ(Helec + Htherm)/Eh - contains an additional correction for RT (H = E + RT).
Σ(Gelec + Gtherm)/Eh - contains the entropic contributions to the free energy (G = H - TS)
Temperature Corrections
# freq=(readisotopes) rb 3lyp/6-31g(d) geom=connectivity
The thermochemistry of anti 2 was recalculated under 0 K.
Optimising Transition States
In terms of the energy diagram the minima, i.e. the products, and the reactants (in this case as the investigation is on a rearrangement), were determined in the optimisation of the conformers of 1,5-hexadiene.

To optimise the transition states of the rearrangement, the two possible transition states were possible: chair and boat. To do this an allyl fragment (CH2CHCH2) was optimised and orientated in the correct positions for the two transition states and optimised to determine the guess structures for the two transition states.
Chair TS - TS(Berny) Optimisation
opt=(calcfc,ts,noeigen freq hf/3-21g geom=connectivity
The fragments on the guess structure were positioned approximately 0.220 nm apart angled at 109.5o between the two terminal ends.
The optimisation seemed successful. There was an imaginary vibrational frequency at -817.86 cm-1. The vibrational mode may be found here. The full optimisation may be found here DOI:10042/to-10093 .
The distance between the terminal ends had shortened to 0.202 nm.
Chair TS - Frozen Coordinate Method
opt=(calcfc,modredundant) rhf/3-21g geom=connectivity
In this method, the terminal ends were "frozen" (i.e. fixed) to 0.220 nm using the Redundant Coord Editor on GaussView and optimised. Then when the job had finished, the fragments were "unfrozen" and optimised again. ( DOI:10042/to-10167 DOI:10042/to-10168 )
The distance between the two terminal ends was 0.220 nm for the frozen structure and 0.202 nm for the unfrozen structure. The final conformations were the same indicating both methods were in agreement with each other.
Boat TS - QST2 Method
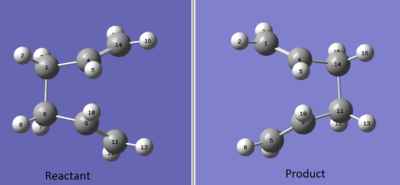
For this investigation two anti 2 conformers were taken and the atoms were labelled fully for both Reactant and Product. For the boat transition state to be located the central C-C-C-C dihedral angle was changed to 0o, and the central C-C-C were reduced to 100o.
After the atoms were orientated in a specific way the structures were optimised using :
opt=qst2 freq hf/3-21g geom=connectivity
There was an imaginary vibrational frequency at -840.08 cm-1. The vibrational mode may be found here. The full optimisation may be found here DOI:10042/to-10101 .
The distance between the two terminal ends was found to be 0.214 nm, slightly further apart than the chair transition state.
Discussion
The symmetry of the transition states were C2h (Chair TS) and C2v (Boat TS) respectively.
Intrinsic Reaction Coordinate Analysis
To check that the optimised transition states above had optimised fully the intrinsic reaction coordinate calculations were run which uses the imaginary vibrational mode found in the frequency analysis above (showing the Cope Rearrangement reaction pathway) and determines the energy stability in steps. This gives a good indication on whether the transition state is at a maximum of an energy diagram.
Chair Analysis
# irc=(forward, maxpoints=50,calcfc) ram1 geom=connectivity
GaussView predicted the following IRC pathway.
| Final Conformation | Total Energy | RMS Gradient |
|---|---|---|
 |
 |
 |
It was fairly obvious looking at the energy diagram that although the transition state was at the maximum, no enough steps had been determined in the forward direction and that the graph did not reach an asymptote. Hence the code was changed so that the force constants would be determined at every step.
# irc=(forward, maxpoints=50,calcall) ram1 geom=connectivity
| Final Conformation | Total Energy |
|---|---|
| DOI:10042/to-10159 | 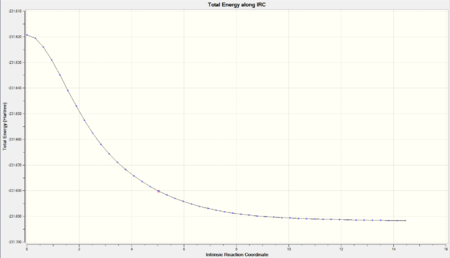 |
This time the energy path converged to an asymptote, which structural and energy analysis determined to be Gauche 2 (from the 1,5-hexadiene conformers).
Boat
# irc=(forward, maxpoints=50,calcfc) ram1 geom=connectivity
| Final Conformation | Total Energy | RMS Gradient |
|---|---|---|
 |
 |
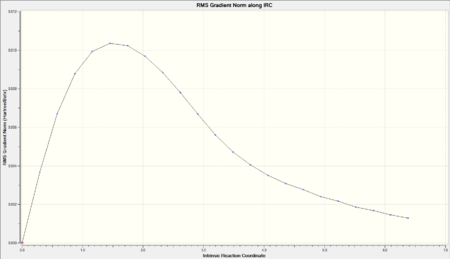 |
A similar problem encountered for the IRC analysis of the boat transition state. Again, "calcall" was used however that proved unsuccessful. Thus the maxpoints was increased to 100.
# irc=(forward, maxpoints=100,calcall) ram1 geom=connectivity
| Final Conformation | Total Energy |
|---|---|
| DOI:10042/to-10160 | 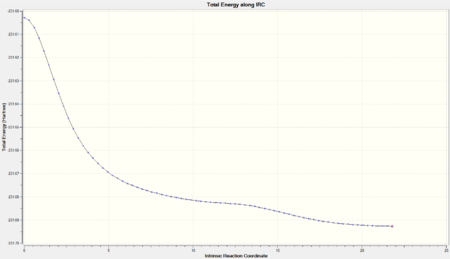 |
The energy path converged to an asymptote, which structural and energy analysis determined to be Gauche 3 (from the 1,5-hexadiene conformers).
Activation Energies
The activation energies were determined by taking the difference in energy, i.e. ΔE, between the energies of the transition state (the maximum) and the energies of anti 2 (the minimum). The sum of electronic and zero-point energies gave the energy difference, i.e. the activation energy, at 0 K and the sum of electronic and thermal energies gave the energy difference at 298.15 K. (The conversion of 1 Eh = 627.509 kcal/mol was used). The energy values for Anti 2 were used as suggested in the script.
| HF/3-21g: ChairDOI:10042/to-10153 and BoatDOI:10042/to-10152 TS | DFT B3LYP/6-31 G*: ChairDOI:10042/to-10158 and BoatDOI:10042/to-10157 TS | |||||
|---|---|---|---|---|---|---|
| Transition State | Electronic Energy/Eh | Sum of Electronic and Zero-Point Energies/Eh | Sum of Electronic and Thermal Energies/Eh | Electronic Energy/Eh | Sum of Electronic and Zero-Point Energies/Eh | Sum of Electronic and Thermal Energies/Eh |
| Chair TS | -231.61932 | -231.46670 | -231.46134 | -234.55698 | -234.41493 | -234.40901 |
| Boat TS | -231.60280 | -231.45093 | -231.44530 | -234.54309 | -234.40234 | -234.39601 |
| Reactant (Anti 2) | -231.69254 | -231.53954 | -231.53257 | -234.61170 | -234.46921 | -234.46186 |
| HF/3-21g | DFT B3LYP/6-31G* | Lit.[2] | |||
|---|---|---|---|---|---|
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | at 0 K | |
| ΔE (Chair) | 45.71 | 44.70 | 34.06 | 33.16 | 33.5 ± 0.5 |
| ΔE (Boat) | 55.60 | 54.76 | 41.96 | 41.32 | 44.7 ± 2.0 |
The values obtained for the activation energies agreed with the literature. It was believed the DFT B3LYP/6-31G* method with basis set was sufficiently accurate.
Discussion
The chair transition state converged/minimised to a Gauche 2 and the boat transition state converged to a Gauche 3. It was considered after activation energies were derived that perhaps ΔE should be calculated from the energy values obtained for Gauche 2 (for chair TS) and Gauche 3 (for boat TS) rather than Anti 2.
| Transition State | Sum of Electronic and Zero-Point Energies/Eh | Sum of Electronic and Thermal Energies/Eh |
|---|---|---|
| Chair TS | -234.41493 | -234.40901 |
| Reactant (Gauche 2) | -234.46821 | -234.46094 |
| Boat TS | -234.40234 | -234.39601 |
| Reactant (Gauche 3) | -234.46849 | -234.46146 |
Thus the activation energies were 33.43 (0 K) and 32.59 (298.15 K) for the Chair TS and 41.51 (0 K) and 41.07 (298.15 K). These were not as accurate as the previous results obtained using anti 2. This was interesting as no free rotation would occur at absolute zero and thus the calculated minima (conformers) would be expected.
It was also worthwhile mentioning that the length between the terminal ends are different for HF calculations than DFT.
| Transition State | Computational Method | Distance /nm |
|---|---|---|
| Chair | HF | 0.202 |
| Chair | DFT | 0.197 |
| Boat | HF | 0.214 |
| Boat | DFT | 0.221 |
The Diels-Alder Cycloaddition
Introduction
Cis-butadiene and ethylene react via a [4πs + 2πs] Diels-Alder Cycloaddition to produce cyclohexene. The transition states in this investigation was found using AM1 semi-empirical calculations.
Frontier Orbitals of the Fragments
| Cis-butadiene HOMO | Cis-butadiene LUMO |
|---|---|
 |

|
| Ethylene HOMO | Ethylene LUMO |
 |

|
The HOMO of ethylene and the LUMO of cis butadiene were symmetric with repect to the vertical plane of symmetry whereas the LUMO of ethylene and the HOMO of cis butadiene were antisymmetric.
Transition State Optimisation
opt=(calcfc,ts,noeigen) freq am1 geom=connectivity
The transition state was calculated.
There was an imaginary frequency at -955.59cm-1 which showed a syn- reaction pathway. (View hereDOI:10042/to-10141 ).
Low real frequencies did not show the reaction pathway with certainty (147.12 cm-1 showed wagging, 246.64 cm-1 showed rocking) but a vibrational mode at 272.37 cm-1 showed certain elements of the reaction path.
The two partly formed C-C σ bonds were found to be 0.212 nm long.
A DFT level calculation was also run.
# opt=(calc,ts) freq rb31yp/6-31g(d) geom=connectivity
Discussion on sp3 and sp2
IRC
# irc=(forward,maxpoints=100,calcall) ram1 geom=connectivity
# irc=(reverse,maxpoints=100,calcall) ram1 geom=connectivity
| Element | ReverseDOI:10042/to-10143 | ForwardDOI:10042/to-10142 |
|---|---|---|
| Product Conformation | Distance between fragments = 0.212 nm | Distance between fragments = 0.152 nm |
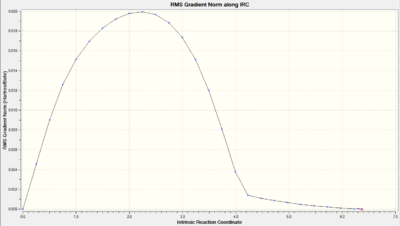
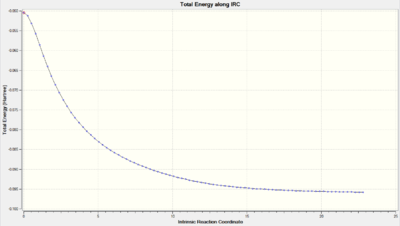
| Total Energy along IRC | 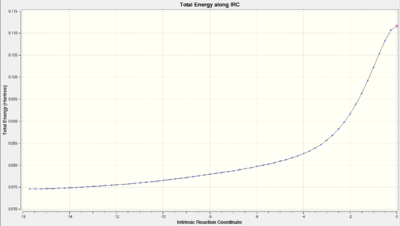 |

|
| RMS Gradient along IRC | 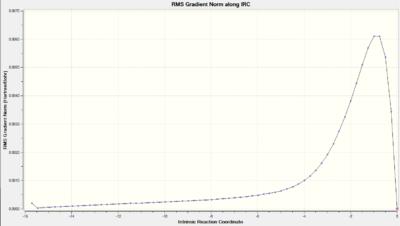 |
|
As we can observe from the Total Energy along the IRC, the transition state was indeed at the maximum on the energy graph. It was also worth noting that the shape of the energy curve suggests a fast optimisation to the transition state but quite a slow bond-making step (i.e. the process of the π orbitals of the dienophile forming two new σ bonds with the π orbitals of the diene).
MO of TS
| HOMO (-0.324) | LUMO (0.023) |
|---|---|
 |

|
| System | Molecular Orbital | Symmetry in the Horizontal Plane | Symmetry in the Vertical Plane |
|---|---|---|---|
| HOMO of TS |  |
Antisymmetric | Antisymmetric |
| LUMO of TS |  |
Antisymmetric | Symmetric |
The HOMO of the transition state has been made from the HOMO of cis butadiene and the LUMO of ethylene whereas the LUMO of the transition state has been made from the LUMO of cis butadiene and the HOMO of ethylene.
The information agreed with the symmetries: the HOMO of TS is antisymmetric made from two antisymmetric fragments, the LUMO of TS is symmetric made from two symmetric fragments.
The Study of the Regioselectivity of the Diels-Alder Reaction
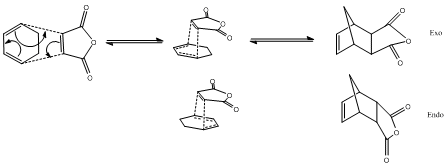
The cycloaddition of cyclohexa-1,3-diene with maleic anhydride produces two isomers, endo and exo. The primary product is the endo adduct. The reaction is supposedly under kinetic control thus the exo transition state is believed to be higher in energy.
The Optimisation of the Transition States
The optimisation was run using the AM1 method.
| Conformation | Imaginary Frequency (cm-1) | Vibrational Motion | Visualisation | DOI |
|---|---|---|---|---|
| Endo | -806.38 |  |
Visualise | DOI:10042/to-10144 |
| Exo | -812.21 |  |
Visualise | DOI:10042/to-10145 |
IRC Analysis
IRC was run to check for the completion of the optimisation of the transition states.
| Isomer | Reverse: Total Energy | Forward: Total Energy | Initial Conformation (Reverse) | Final Conformation (Forward) |
|---|---|---|---|---|
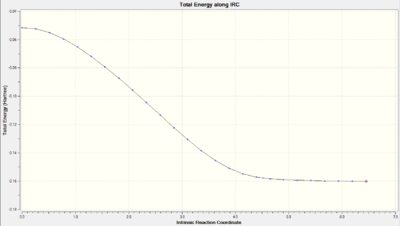
| Endo | 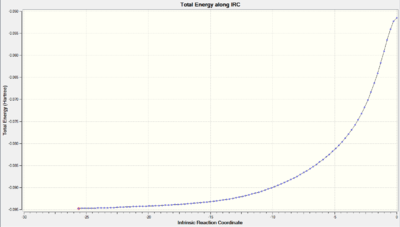 DOI:10042/to-10147 DOI:10042/to-10147 |
 DOI:10042/to-10146 DOI:10042/to-10146 |
||
| Exo | 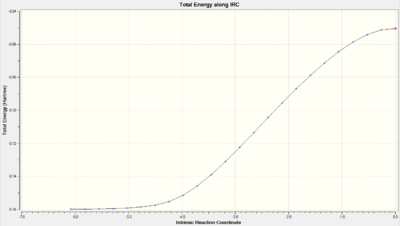 DOI:10042/to-10148 DOI:10042/to-10148 |
 DOI:10042/to-10149 DOI:10042/to-10149 |
The transition states were indeed at the maxima however the curves produced were interestingly not of the same shape. The IRC energy path of the exo seemed to proceed in different directions (i.e. forward for reverse and reverse for forward).
Relative Energies
The relative energies of the transition states were as follows - the end products were also observed:
| Isomer | Relative Energy (AM1)/Eh | Isomer End Product | Relative Energy (AM1)/Eh | |
|---|---|---|---|---|
| Endo | -0.051505 | Endo End Product (IRC Forward: Step 26) | -0.160170 | |
| Exo | -0.050420 | Exo End Product (IRC Forward: Step 24) | -0.159909 |
The endo transition state was lower in energy (i.e. more stable) as well as the endo end product (obviously the transition state being much less stable than the end product). The end products do not differ in energy by a large amount (0.163780 kcal/mol) but the transition state relative energies differ by 0.680845 kcal/mol. This showed that the the endo form was more kinectically, and thermodynamically stable.
MO Analysis
| Isomer | HOMO Front | HOMO Back | LUMO Front | LUMO Back |
|---|---|---|---|---|
| Endo (HOMO -0.345) (LUMO -0.036 ) |  |
 |
 |

|
| Exo (HOMO -0.343) (LUMO -0.040) |  |
 |
 |

|
The HOMO and the LUMO of the two transition states looked fairly similar however there were some major differences.
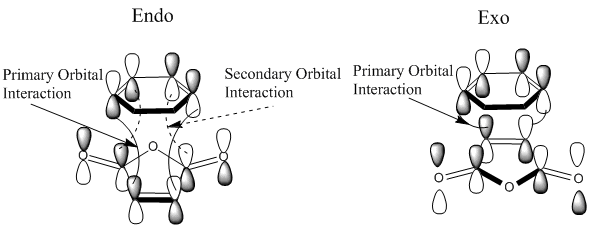
Secondary Orbital Interactions
| Isomer | Front | Side |
|---|---|---|
| Endo 0.029 | 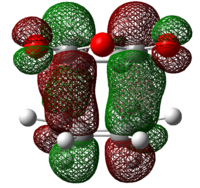 |
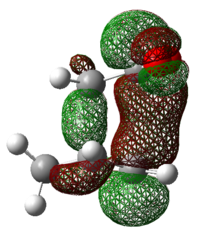
|
| Exo 0.034 | 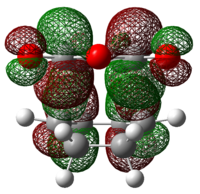 |
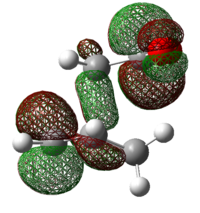
|
Secondary Orbital Interactions (SOI) were found in the LUMO+2 of the transition states. As seen from the diagram above, the endo transitions has SOI but not exo[3].
Further Discussion
Conclusion
References
- ↑ O. Wiest, K.A. Black, K.N. Houk, "Density Functional Theory Isotope Effects and Activation Energies for the Cope and Claisen Rearrangements", J. Am. Chem. Soc., 1994, 116, 10336-10337DOI:10.1021/ja00101a078
- ↑ M.J. Goldstein, M.S. Benzon, "Boat and chair transition states of 1,5-hexadiene", J. Am. Chem. Soc., 1972, 94(20), 7147-7149, DOI:10.1021/ja00775a046
- ↑ A. Arrieta, F.P. Cossio, "Direct Evaluation of Secondary Orbital Interactions in the Diels-Alder Reaction between Cyclopentadiene and Maleic Anhydride", J. Org. Chem., 2001, 66(18), 6178-6180DOI:10.1021/jo0158478