Rep:Mod:hl36111C
Experiment 1C: Advanced molecular modelling and assigning the absolute configuration of an epoxide
Part One
The Hydrogenation of Cyclopentadiene Dimer
The dimerisation of cyclopentadiene and the hydrogenation of the preferred isomer has been investigated. The molecules were produced on chem draw and then optimised using a MMFF94(s) force field and the total energy calculated.
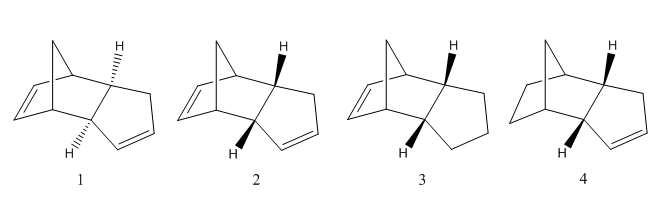
Molecules 1 and 2 are the isomers of the cyclopentadiene dimer. Isomer 1 is the exo configuration and isomer 2 is the endo configuration. Molecules 3 and 4 are the possible products of hydrogenation of the endo isomer.
| Energy Type/ kcal/mol | Isomer 1 (Exo)
|
Isomer 2 (Endo)
|
Isomer 3
|
Isomer 4
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total bond stretching energy | 3.543 | 3.468 | 3.312 | 2.823 | ||||||||||||
| Total angle bending energy | 30.773 | 33.193 | 31.920 | 24.686 | ||||||||||||
| Total torsional energy | -2.731 | -2.949 | -1.454 | -0.378 | ||||||||||||
| Total van der Waals energy | 12.802 | 12.356 | 13.637 | 10.637 | ||||||||||||
| Total electrostatic energy | 13.014 | 14.183 | 5.119 | 5.147 | ||||||||||||
| Total Energy | 55.373 | 58.191 | 50.446 | 41.257 |
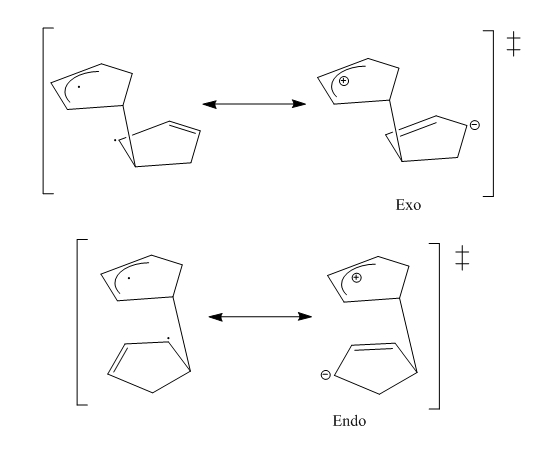
The dimerisation of cyclopentadiene produces the endo dimer (2) rather that the exo dimer (1). From the table the total energies show that the endo dimer (58.19 kcal/mol) is higher in energy that the exo dimer (55.37 kcal/mol). Hence suggesting that the dimerisation of cyclopentadiene is under kinetic control as the least stable dimer forms. This is due to the endo transition state being favoured over the exo. This is the result of a combination of factors. In the endo conformation there is a plane to plane orientation of diene to dienophile.[1] The endo transition state has orbital symmetry and thus creating secondary orbital interactions in the endo configuration.[2]In the asynchronous mechanism, the transition state is a zwitter ion. For the endo isomer, the charges are stabilised more than for the exo isomer. Thus resulting in the endo product being formed. The transition states can be seen below:
The hydrogenation of cyclopentadiene results in either molecule 3 or 4. Further hydrogenation leads to the tetrahydro derivative. It can be seen from the table that the isomer with the lowest energy is number 4 and hence this is the most thermodynamically favoured product. Therefore being the most likely isomer to be formed as the reaction is under thermodynamic control.
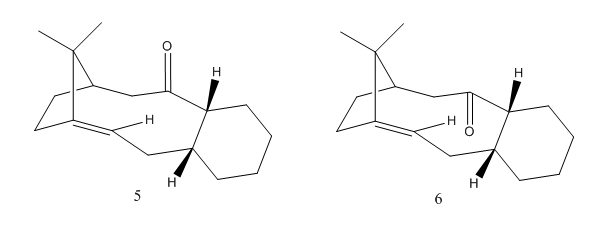
The molecules below are two isomers of the carbonyl intermediate in the synthesis of Taxol ( an important drug in the discovery of ovarian cancer.) The two isomers show atropisomerism due to restricted rotation around a single bond. During the reaction one isomer is produced first and then after standing for a while, transforms to the other isomer. The table below displays the calculated energies using the MMFF94(s) force field.
| Energy Type/ kcal/mol | Isomer 5
|
Isomer 6
| ||||||
|---|---|---|---|---|---|---|---|---|
| Total bond stretching energy | 7.688 | 7.685 | ||||||
| Total angle bending energy | 28.332 | 16.903 | ||||||
| Total torsional energy | 0.174 | 2.107 | ||||||
| Total van der Waals energy | 33.146 | 33.957 | ||||||
| Total electrostatic energy | 0.301 | -0.061 | ||||||
| Total Energy | 70.544 | 61.188 |
From the computational results, it can be seen that isomer 6 is lower in energy than isomer 5. Hence, isomer 6 is the most stable and therefore the thermodynamic product. From this we can deduce that isomer 5 may be produced first (the kinetic product) and after being left to stand it converts to the thermodynamic product, isomer 6. The lower energy of isomer 5 may be due to the carbonyl group pointing upwards. In this position it is more sterically hindered as it is close to the methyl groups. Resulting in a less stable configuration.
The alkene can be described as “hyperstable” because it contains less strain than the parent hydrocarbon. This means that the alkene slowly reacts. The alkene is located at the bridgehead carbon and the cage structure of the olefin adds stability. The strain associated with the alkene at the bridgehead carbon results in a decrease in the HOMO-LUMO energy gap and hence the alkene is more stable. [3]
Taxol Derivative
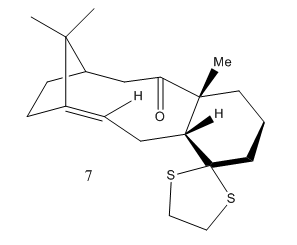
Molecule 7 below is a derivative of isomer 6 above. The table below displays its energy information. The 1H and 13C NMR has been produced and compared to literature values.
| Energy Type/ kcal/mol | Isomer 7
| |||
|---|---|---|---|---|
| Total bond stretching energy | 14.997 | |||
| Total angle bending energy | 30.960 | |||
| Total torsional energy | 9.670 | |||
| Total van der Waals energy | 49.330 | |||
| Total electrostatic energy | -6.020 | |||
| Total Energy | 100.497 |
The NMR files are linked to:DOI:10042/26532
1H NMR (solvent=benzene)
| Computational/ ppm | Literature/ ppm [4] |
|---|---|
| 5.98 (1 H) | 5.21 (m,1 H) |
| 3.20 (2 H) | 3.00-2.70 (m, 6 H) |
| 3.06 (1 H) | |
| 2.97 (1 H) | |
| 2.77 (3 H) | |
| 2.66 (1 H) | 2.70- 2.35 (m, 4 H) |
| 2.48 (3 H) | |
| 2.33 (1 H) | 2.20-1.70 (m, 6 H) |
| 2.23 (1 H) | |
| 2.01 (3 H) | |
| 1.84 (1 H) | 1.58 (t, J = 5.4 Hz, 1 H) |
| 1.58 (4 H) | 1.50-1.2 (m, 3 H) |
| 1.28 (3 H) | 1.10 (s, 3 H) |
| 1.21 (1 H) | |
| 0.96 (3 H) | 1.07 (s, 3 H) |
| 0.64 (1 H) | 1.03 (s, 3 H) |
Comparing the 1H NMR shifts is difficult as the literature has grouped the shifts. Looking at the overall picture of the NMR, the shifts are at similar ppm values and hence suggesting that the computed NMR has been correctly assigned.
13C NMR (solvent=benzene)
| Computational/ ppm | Literature/ ppm [4] |
|---|---|
| 212.12 | 211.49 |
| 147.97 | 148.72 |
| 120.03 | 120.90 |
| 94.01 | 74.61 |
| 60.32 | 60.53 |
| 54.78 | 51.30 |
| 54.09 | 50.94 |
| 49.59 | 45.53 |
| 49.04 | 43.28 |
| 46.67 | 40.82 |
| 41.69 | 38.73 |
| 41.69 | 36.78 |
| 38.64 | 35.47 |
| 34.03 | 30.84 |
| 33.54 | 30.00 |
| 28.05 | 25.56 |
| 26.36 | 25.35 |
| 24.44 | 22.21 |
| 22.51 | 21.39 |
| 21.61 | 19.83 |
Looking at the computational results and the data from literature it can be seen that the compound has been correctly assigned. Some of the values are very similar for example the shift at 120.03 is extremely close to that of 120.90. However, there are others that do not correspond as well, 94.01 and 74.01. These could be due to spin-orbit coupling errors. The carbons attached to heavier elements such as sulphur need to be corrected.
The energies of the molecule can also be obtained:
Zero-point correction= 0.467823 (Hartree/Particle) Thermal correction to Energy= 0.489247 Thermal correction to Enthalpy= 0.490191 Thermal correction to Gibbs Free Energy= 0.421084 Sum of electronic and zero-point Energies= -1651.416520 Sum of electronic and thermal Energies= -1651.395096 Sum of electronic and thermal Enthalpies= -1651.394152 Sum of electronic and thermal Free Energies= -1651.463259
The sum of electronic and thermal free energies is also the free energy ΔG. This can be used when comparing to isomers.
Part Two
Shi Pre-Catalyst
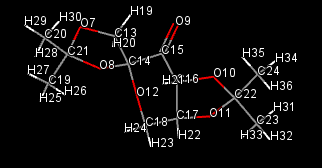
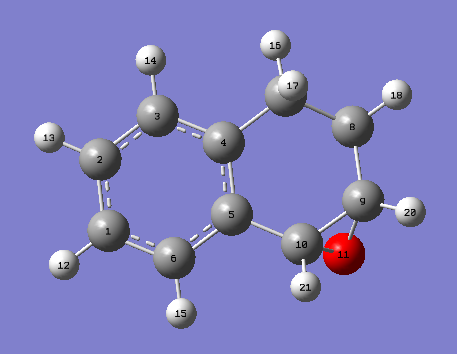
Using the mercury program, the bond distances of anomeric centres (O-C-O) can be viewed. The image below displays the labelled atoms and the following table displays the bond lengths.
| Atom 1 | Atom 2 | Bond Length |
|---|---|---|
| O8 | C14 | 1.405(4) |
| C14 | O12 | 1.420(4) |
| O7 | C21 | 1.406(4) |
| C21 | O8 | 1.460(4) |
| O10 | C22 | 1.442(4) |
| C22 | O11 | 1.430(4) |
The anomeric effect occurs when the oxygen lone pair can overlap with an anti-periplanar antibonding orbital of the second C-O bond. This shortens the length of the donating oxygens' O-C bond and lengthens the second C-O bond.This can be seen in the examples above in the table. This can be seen in the first two sets of values in the table.
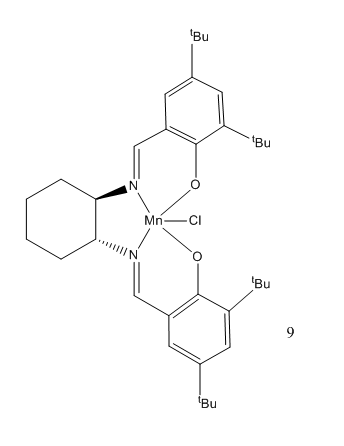
Jacobsen Pre-Catalyst
There are two tBu groups present on each of the phenyl rings in the molecule. The tBu groups are bulky and in this case in close proximity to each other. This prevents the alkene approaching the catalyst at this side of the molecule and hence promotes the approach of the alkene to the diimine bridge.[5] Therefore, allow for a more successful epoxidation reaction to occur.
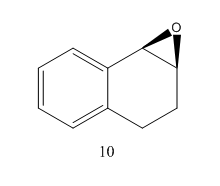
1,2-dihydronaphthalene oxide
One enantiomer of 1,2-dihydronaphthalene oxide was produced, optimised and energies calculated.
| Energy Type/ kcal/mol | Epoxide 10
| |||
|---|---|---|---|---|
| Total bond stretching energy | 2.698 | |||
| Total angle bending energy | 2.180 | |||
| Total torsional energy | 1.674 | |||
| Total van der Waals energy | 19.379 | |||
| Total electrostatic energy | 5.153 | |||
| Total Energy | 30.224 |
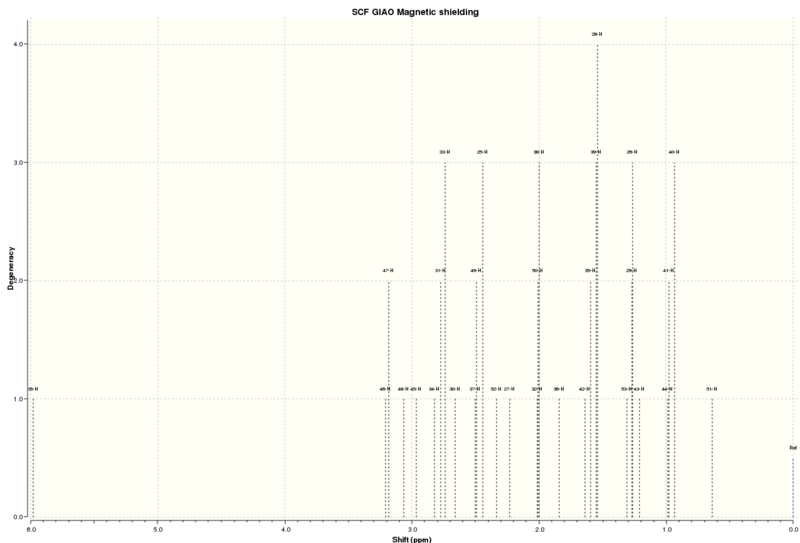
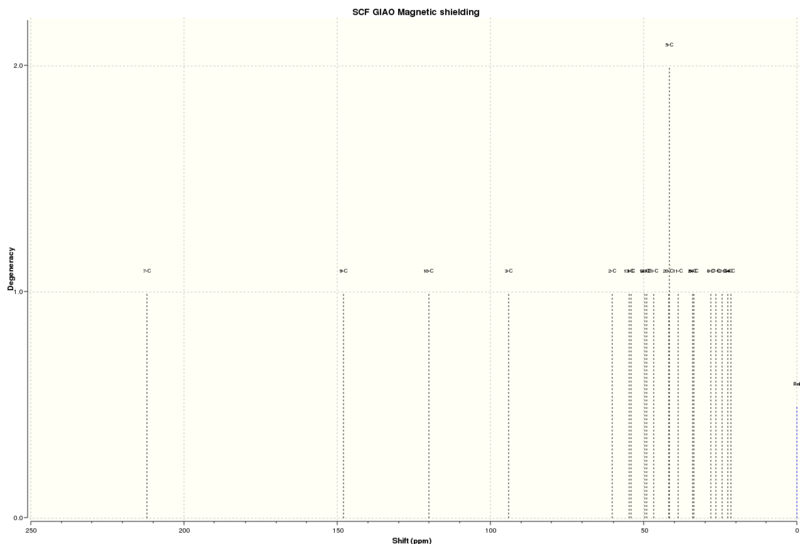
NMR
The 1H and 13C NMR was produced for the molecule.
The NMR files are linked to:DOI:10042/26600
Coupling
The NMR coupling files are linked to:DOI:10042/26774
13C NMR (solvent=chloroform)
| Atom | Computational/ppm | Literature/ppm [6] |
|---|---|---|
| 4 | 135.39 | 136.7 |
| 5 | 130.37 | 132.6 |
| 6 | 126.67 | 129.5 |
| 2 | 123.79 | 128.4 |
| 3 | 123.53 | 128.4 |
| 1 | 121.74 | 126.1 |
| 9 | 52.82 | 55.1 |
| 10 | 52.19 | 52.7 |
| 7 | 30.18 | 24.4 |
| 8 | 29.06 | 21.8 |
The 13C chemical shifts calculated using Gaussview are a close match to those found in the literature. Therefore, it can be assumed that the NMR has been correctly assigned. The carbons of the phenyl ring have higher chemical shifts than those close to the epoxide functional group. This is due to the deshielding of the benzine ring, raising the values of the chemical shifts.
1H NMR (solvent=chloroform)
| Atom | Computational/ppm | Literature/ppm [6] |
|---|---|---|
| 15 | 7.62, 1H | 7.28, 1H |
| 13, 12 | 7.39, 2H | 7.08-7.17, 2H |
| 14 | 7.25, 1H | 6.98, 1H |
| 21 | 3.56, 1H | 3.74, 1H |
| 20 | 3.48, 1H | 3.62, 1H |
| 17 | 2.95, 1H | 2.68, 1H |
| 16 | 2.27, 1H | 2.44, 1H |
| 18 | 2.21, 1H | 2.30, 1H |
| 19 | 1.87, 1H | 1.65, 1H |
The 1H NMR chemical shifts are close to the literature values. Therefore, showing that the NMR has been correctly assigned via computational calculations. The hydrogens of the benzene ring have higher chemical shifts because of deshielding.
Optical Rotation
The optical rotation for the enantiomer has been calculated.
The OR files are linked to:DOI:10042/26622
[Alpha] ( 5890.0 A) = -35.86 deg.
Literature values:
(1R,2S):[α]D=+133° (C=-0.39,CHC13)[7]
(1S,2R):[α]D=-133° (C=0.21,CHC13)[7]
From the literature values it appears that the 1S,2R epoxide has been computed as they both have negative values for optical rotation. The values are very different, suggesting that either the computational calculated value or the literature values could be incorrect.
Electronic Circular Dichroism
An ECD spectrum was produced for the enantiomer above. However, it does not show anything useful for assigning the epoxides, as no appropriate chromophore exists.
The ECD files are linked to:DOI:10042/26603
Vibrational Circular Dichroism
A VCD spectrum of the epoxide was produced.
Transition States of the Epoxidation using the Shi Catalyst
The energies below are for the transition states of the epoxidation of 1,2-dihydronaphthalene using the Shi catalyst.
R,S epoxide
1) sum of electronic and thermal free energies= -1381.120782 Hartrees 2) sum of electronic and thermal free energies= -1381.125886 Hartrees 3) sum of electronic and thermal free energies= -1381.134059 Hartrees 4) sum of electronic and thermal free energies= -1381.126722 Hartrees
S,R epoxide
1) sum of electronic and thermal free energies= -1381.131343 Hartrees 2) sum of electronic and thermal free energies= -1381.116109 Hartrees 3) sum of electronic and thermal free energies= -1381.126039 Hartrees 4) sum of electronic and thermal free energies= -1381.136239 Hartrees
The lowest energy transition state for each enantiomer has been used to calculate the enantiomeric excess:
| Epoxide | Energy/ Hartrees | Energy/ kJ/mol |
|---|---|---|
| R,S | -1381.134059 | -3626166.091 |
| S,R | -1381.136239 | -3626171.814 |
| Difference | ΔG/ kJ/mol | lnK | K | (K/(1+K))x100/ % |
|---|---|---|---|---|
| (R,S-S,R) | 5.72358782 | -2.308869459 | 0.099 | 9.04 |
| (S,R-R,S) | -5.72358782 | 2.308869459 | 10.063 | 90.96 |
enantiomeric excess= 90.96-9.04= 81.93% (1S,2R)
From these results it can be seen that the 1S,2R epoxide is in enantiomeric excess over the 1R,1S epoxide. Therefore suggesting it is the preferred isomer. This can also be backed up by looking at the energy, as the S,R epoxide transition state has a lower energy than the R,S.
Styrene oxide
One enantiomer of styrene oxide has been optimised and the energy calculated. Further analysis has also been performed.
| Energy Type/ kcal/mol | Isomer 11
| |||
|---|---|---|---|---|
| Total bond stretching energy | 1.84162 | |||
| Total angle bending energy | 1.42456 | |||
| Total torsional energy | 2.34617 | |||
| Total van der Waals energy | 13.67228 | |||
| Total electrostatic energy | 3.07530 | |||
| Total Energy | 21.61504 |
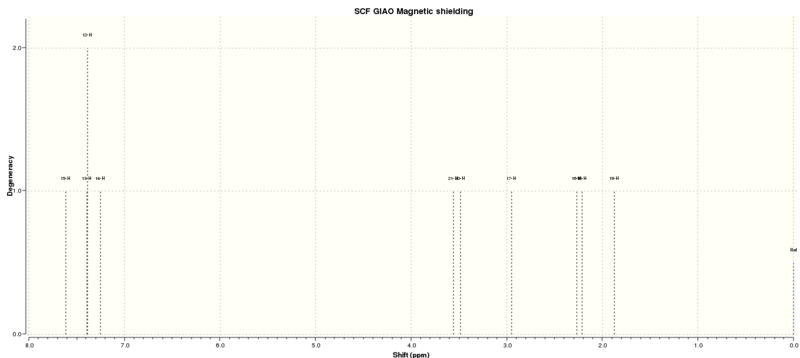
NMR
The NMR files are linked to:DOI:10042/26601
Coupling
The NMR coupling files are linked to:DOI:10042/26775
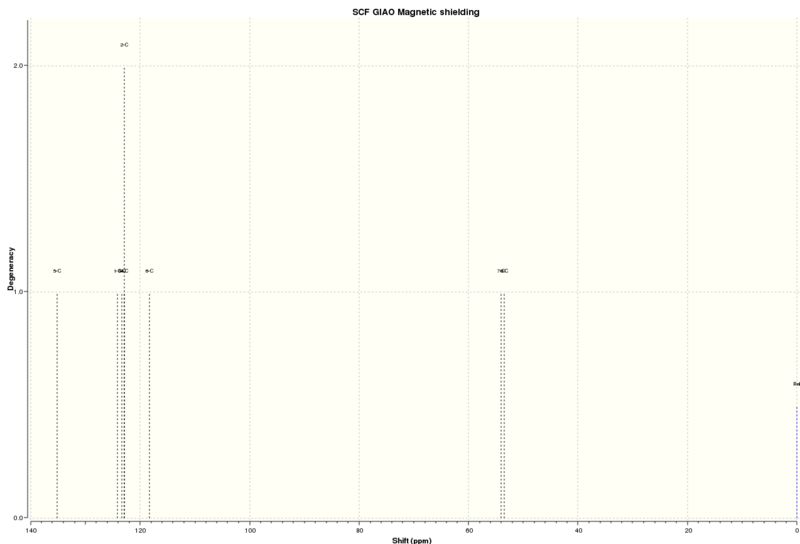
13C NMR (solvent=chloroform)
| Atom | Computational/ppm | Literature/ppm[8] |
|---|---|---|
| 5 | 135.13 | 137.6 |
| 1 | 124.13 | 128.5 |
| 3 | 123.41 | 128.5 |
| 4 | 122.96 | 128.1 |
| 2 | 122.96 | 125.5 |
| 6 | 118.27 | 125.5 |
| 7 | 54.06 | 52.4 |
| 8 | 53.47 | 51.2 |
The 13C NMR chemical shifts obtained are close to those found in literature, suggesting that the NMR has been calculated correctly. The carbons of the epoxide functional group have lower chemical shifts than those in the the phenyl ring, due to deshielding causing higher shifts.
1H NMR (solvent=chloroform)
| Atom | Computational/ppm | Literature/ppm[8] |
|---|---|---|
| 13, 10, 12, 11 | 7.49, 4H | 7.34-7.27, 5H |
| 14 | 7.30, 1H | |
| 15 | 3.66, 1H | 3.84, 1H |
| 16 | 3.12, 1H | 3.13, 1H |
| 17 | 2.54, 1H | 2.81, 1H |
The computational 1H chemical shifts are similar to the literature values. Therefore suggesting that the NMR has been correctly assigned. The hydrogens of the phenyl ring have high chemical shifts than those close to the epoxide. This is due to the benzene ring being deshielding and hence the chemical shift is higher.
Optical Rotation
The optical rotation for the enantiomer above was calculated.
The optical rotation files are linked to:DOI:10042/26604
[Alpha] ( 5890.0 A) = -30.44 deg.
Literature values:
R enantiomer: [α]D21= -24 (c 1.00, CHCl3)[9]
S enantiomer: [α]D21= +24 (c 1.00, CHCl3)[9]
From the literature values it can be seen that the computational calculations have been performed on the R enatiomer of styrene oxide. The optical rotation for this enantiomer is a negative value and hence corresponds to the negative literature value. The literature values are not extremely close. However, close enough to suggest that the correct molecule has been assigned.
Electronic Circular Dichroism
The ECD spectrum has been created. However for the epoxides, the appropriate chromophore is not presentand hence it is not useful in assigning the absolute configuration of the molecule.
The ECD files are linked to:DOI:10042/26602
Vibrational Circular Dichroism
The VCD spectrum has been created.
Transition States of the Epoxidation Reaction using the Shi Catalyst
The energy values below are for the transition states of the epoxidation of styrene using the shi catalyst:
R epoxide
1) sum of electronic and thermal energies= -1303.730703 Hartrees 2) sum of electronic and thermal energies= -1303.730238 Hartrees 3) sum of electronic and thermal energies= -1303.736813 Hartrees 4) sum of electronic and thermal energies= -1303.738044 Hartrees
S epoxide
1) sum of electronic and thermal energies= -1303.733828 Hartrees 2) sum of electronic and thermal energies= -1303.724178 Hartrees 3) sum of electronic and thermal energies= -1303.727673 Hartrees 4) sum of electronic and thermal energies= -1303.738503 Hartrees
The lowest energy transition state for the R and S epoxides have been used to calculate the enantiomeric excess:
| Epoxide | Energy/ Hartrees | Energy/ kJ/mol |
|---|---|---|
| R | -1303.738044 | -3422962.931 |
| S | -1303.738503 | -3422964.136 |
| Difference | ΔG/ kJ/mol | lnK | K | (K/1+K)x100/% |
|---|---|---|---|---|
| (R-S) | 1.205104041 | -0.486133524 | 0.615 | 38.08 |
| (S-R) | -1.205104041 | 0.486133524 | 1.626 | 61.92 |
Enantiomeric excess= 61.92-38.08= 23.84% (S)
Literature value=24% [10]
From the calculated results it can be seen that the S epoxide is in enantiomeric excess. This can be backed up when looking at the energies of the transition states. The S epoxide transition state has a lower energy than the R epoxide transition state. Hence, the R epoxide is preferentially formed. The calculate value is also the same as literature value, hence the enantiomeric excess has been successful calculated.
Non-covalent Interaction (NCI) Analysis
The NCI analysis shows non-covalent interactions. The different colours represent how attractive or repulsive the interaction is:
| Colour | Representation |
|---|---|
| Blue | very attractive |
| Green | mildly attractive |
| Yellow | mildly repulsive |
| Red | very repulsive |
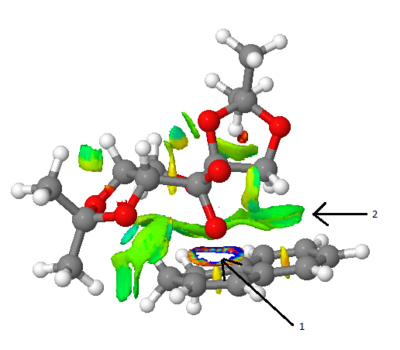
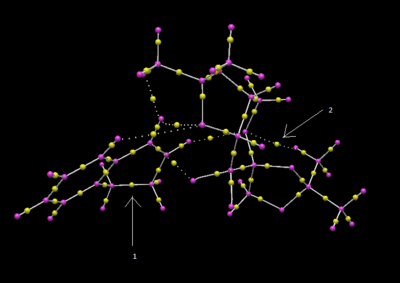
This analysis has been performed on a shi catalyst 1,2-dihydronaphthalene transition state. Arrow 1 points to the bond forming in a transition state, it can be considered half covalent and is usually ignored. Arrow 2 points to a non-covalent interaction between the shi catalyst and the 1,2-dihydronaphthalene. The green colour shows it is mildly attractive.
QTAIM
QTAIM looks at the electron density of both covalent areas (bonds) and non-covalent interactions. The yellow dots on the image represent bond critical points (BCPs). Arrow one represents a BCP of a non-covalent interaction where as arrow 2 represents a BCP of a covalent bond. The BCPs can be at different points relative to the two atoms it connects. For example, the BCP of arrow one is half way between the two atoms, where as the BCP for arrow 2 is closer to the atom on the right-hand side.
References
- ↑ 1.0 1.1 http://www.ch.ic.ac.uk/motm/porphyrins/introDA.html
- ↑ P. Caramella, P. Quadrelli, and L. Toma, J. Am. Chem. Soc., 2002, 124, 1130-1131.
- ↑ W. F. Maier and P. V. R. Schleyer, J. Am. Chem. Soc., 1981, 103, 1891-1900.
- ↑ 4.0 4.1 L. A. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, and R. D. Rogers, J. Am. Chem. Soc., 1990, 112, 277-283.
- ↑ E. N. Jacobsen, W. Zhang, A. R. Muci, J. R. Ecker, and L. Deng, J. Am. Chem. Soc., 1991, 113, 7063-7064.
- ↑ 6.0 6.1 K. Smith, C.-H. Liu, and G. A. El-Hiti, Org. Biomol. Chem., 2006, 4, 917-27. Cite error: Invalid
<ref>tag; name "DiNap" defined multiple times with different content - ↑ 7.0 7.1 A. J. Blacker, S. M. Brown, D. R. Boyd, and G. N. Sheldrake, 1994.
- ↑ 8.0 8.1 L. Ji, Y.-N. Wang, C. Qian, and X.-Z. Chen, Synth. Commun., 2013, 43, 2256-2264. Cite error: Invalid
<ref>tag; name "NMR3" defined multiple times with different content - ↑ 9.0 9.1 D. C. Forbes, S. V. Bettigeri, S. A. Patrawala, S. C. Pischek, and M. C. Standen, Tetrahedron, 2009, 65, 70-76.
- ↑ M. Hickey, D. Goeddel, Z. Crane, and Y. Shi, Proc. Natl. Acad. Sci. U.S.A., 2004, 101, 5794-8.