Rep:Mod:hgl0901
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Objectives
Computational chemistry enables chemists to accurately model many aspects of organic structure and reactivity. Such techniques are not only important for rationalisations of outcomes of reactions, but also extremely useful for predictions of modifications or even new types of reaction. Some of the diversity of such molecular modelling was attempted in the following experiments.
Modelling using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
Dimerisation of Cyclopentadiene
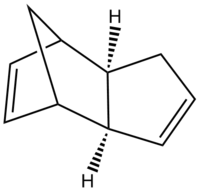
The dimerisation of cyclopentadiene undergoes via Diels-Alder [2+4] cycloaddition, giving two products, the exo 1 and the endo 2 adducts. One molecule of cyclodiene acts as a 4π electron diene and the other one as a 2π electron dienophile. The reaction Scheme is shown in Scheme 1.
The exo adduct is resulted from both σ bonds forming on the bottom face of the diene and both σ bonds forming on the top face of the dienophile, while the endo one is resulted from both σ bonds forming on the bottom faces on the diene and dienophile.[2] This is illustated in Figure 1. It can be seen that apart from the formations of two new σ bonds, at the back of endo dimer, there's also bonding interaction(shown in blue
arrow) since the symmetry of the orbitals are correct. Therefore, the endo 2 is more favoured due to this interaction between the space of the orbitals.[3]

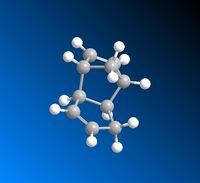 |
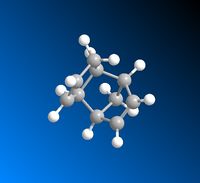 |
However, the energies obtained by applying an MM2 force field to obtain the optimised geometries in Chembio3D suggest the exo dimer is more thermodynamically stable, having a lower total energy than that of the endo dimer. The endo dimer is thus the kinetic product. The results are summarised in Table 1.
As seen from the above table, the slight lower stretching energy of the endo dimer is due to the attractive interaction at the back of the diene, which is negative and resulted as a lower value of 6-12 potential.[4] The main difference of the energies between the two dimers generate from the torsional strain, with a difference of 7.6515 kJ/mol.
Although the exo dimer has a lower total energy than that of the endo dimer, it is reported in the literature[5] the endo dimer is the major product, with a yield of 95% while the exo is on of 5%, which agrees with the frontier molecular orbital approaches obtained. This means that the dimerisation of cyclopentadiene is kinetically controlled, with a lower energy barrier at the transition state.
Hydrogenation of Endo-Cyclopentadiene Dimer
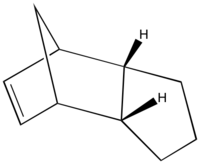
The hydrogenation of the endo dimer of cyclopentadiene yields 3 and 4, which is shown below as Jmol files respectively. The optimised geometries of both are obtained using Chembio3D in MM2 force field. The results are summarised in Table 2.
By comparing the total energies of 3 and 4, it can be seen that 4 is thermodynamically more stable with a much lower energy than that of 3. The main difference between the two is due to the bending energy, caused predominantly by the H-C=C bendings.[6] The torsion energies decrease in a small amount after hydrogenation. The bending energies of both 3 and 4 are lower than that of 2 as a result of the loss of one double bond. A comparison of the bond angles of the Endo-dimer and the hydrogenation products, 3 and 4 are shown in Table 3. The bond angle of H-C=C is 120 degree. [7] However, in all three cases, none of them are at 120 degree, the closer the angle to 120 degree, the less the binding energies required and less bond strain it has. Although both bond angles exhibited are closer than the others to the literature, the presence of two also results as a higher bending energy. Therefore, 4 is the one with the least strain and lowest binding energy. In addition, 1,4 Van der Waals energy also account for the energy difference between the two hydrogenation products.
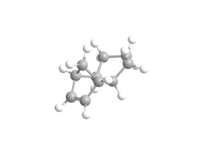 |
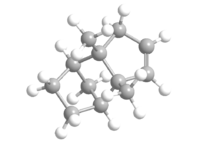 |
| Molecules | H-C=C angle 1 in blue
(degree) |
H-C=C angle 2 in red
(degree) |
Bending Energies (kJ/mol) |
|---|---|---|---|
Endo-dimer |
113 | 108 | 87.2856 |
3 |
N/A | 107 | 78.6327 |
4 |
112 | N/A | 54.4851 |
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
The most Stable Isomer
In the synthesis of Taxol proposed by Paquette, an intermediate 9 or 10 is initially prepared from the carbonyl group pointing either up or down, which are atropisomers of each other.[8]
 |
 |
| Species | 9 | 10 |
|---|---|---|
| Stretching(kJ/mol) | 11.6548 | 10.9694 |
| Bending(kJ/mol) | 69.2631 | 47.4905 |
| Stretch-Bend(kJ/mol) | 1.8016 | -3.4993 |
| Torsion(kJ/mol) | 76.4305 | 82.3384 |
| Non-1,4 Van der Waals(kJ/mol) | -6.5155 | -9.0393 |
| 1,4 Van der Waals(kJ/mol) | 54.8827 | 53.8870 |
| Dipole/Dipole (kJ/mol) | -7.2222 | -8.3824 |
| Total Energy(kJ/mol) | 200.2948 | 178.7056 |
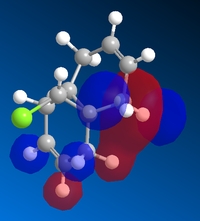
The total energy of isomer 10 is lower than that of 9. The negative contribution from the stretch-bend energy indicates a stretch responding to a reduced angle between two bonds in order to alleviate the strain, which could possibly be due to the hydrogen bonding within the molecule. The much lower bending energy of 10 compared with 9 is a result of having the carbonyl group not pointing on the same side as the hydrogen, thus less hydrogen bonding and less energy is required to put in during optimisation to break the hydrogen bonding interaction.[9] This is illustrated in the Figure 2. The main atoms involved in the hydrogen bonding are highlighted with green. The ones with two pink balls on it are oxygen, and the other one represents for hydrogen. The Jmol files of both intermediates are also included.
 |
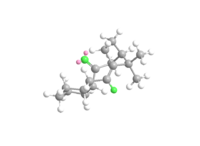 |
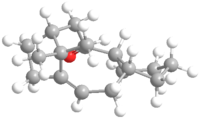 |
The results obtained from the optimisation of geometries in MM2 force field agree with the ones obtained in the MM2 force field as well as the literature. [10] Intermediate 9 is therefore the kinetic product with a lower transition state than that of intermediate 10. Both molecules are optimised by running on SCAN and the optimised intermediate 9 turns out to have a 'twist-boat' for the cyclohexane rather than the 'chair' used for the optimisation of geometries in MM2 force field and MMFF94 force field.
| Intermediates | MM2 Force Fields (kJ/mol) | MMFF94 Force Fields (kJ/mol) |
|---|---|---|
| 9 | 200.2948 | 195.3302 |
| 10 | 178.7056 | 253.5781 |
The slow reaction with alkene
OS, olefinic strain is the extra strain associated with the double bond.[11] It can be used to examine the stability of different species, and heats of hydrogenation is related to it.
In this case, the thermodynamic intermediate 10 is compared with its hydrogenation product. The results are summarised in Table 6. The hydrogenation product has a higher energy than that of the 10, which indicates the 10 is more stable, i.e. low reactivity, belonging to the hyperstable olefins, whose olefin strain energies are negative, less than that of their parent hydrocarbon. [12] The difference between the two is the olefin strain, which is -91.616kJ/mol. [13] The loss of the double bond during hydrogenation results as more strain in the molecule, which can be seen via the Jmol file. Therefore, the reaction with alkene is slow.
| Species | Hydrogenation product of 10 | 10 |
|---|---|---|
| Stretching(kJ/mol) | 3.3587 | 10.9694 |
| Bending(kJ/mol) | 74.4610 | 47.4905 |
| Stretch-Bend(kJ/mol) | 1.8016 | -3.4993 |
| Torsion(kJ/mol) | 106.4753 | 82.3384 |
| Non-1,4 Van der Waals(kJ/mol) | -2.7779 | -9.0393 |
| 1,4 Van der Waals(kJ/mol) | 72.8759 | 53.8870 |
| Dipole/Dipole (kJ/mol) | 0 | -8.3824 |
| Total Energy(kJ/mol) | 270.3216 | 178.7056 |
Modelling Using Semi-empirical Molecular Orbital Theory
Objectives
In this section, the electronic aspects of the molecular reactivity are studied and how they influence on bonds and spectra properties.
Regioselective Addition of Dichlorocarbene
Part 1
In this section, the energy minimisation of 12 is carried out in MM2 force field as well as using Mopac interface/PM6. The results of energies from geometry optimisation is shown in Table 7.
Molecular Orbital Diagrams of Molecule 12 obtained using Mopac/PM6
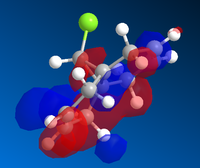 |
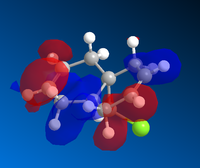 |
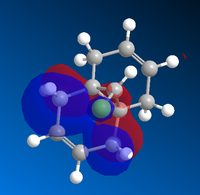 |
 |
Mopac Interface ------------
Model: molecule 12.mol
Mopac Job: AUX PM6 CHARGE=0 EF GNORM=0.100 GRAPH SHIFT=80 Finished @ RMS Gradient = 0.09310 (< 0.10000) Heat of Formation = 19.74008 Kcal/Mol
The molecule 12 has a symmetry of Cs. [15] Only LUMO+1 orbital is symmetrical, lying in the same mirror plane of the molecule. As seen from the above orbitals, most of the electron density is away from the green Cl atom as a result of electrostatic repulsion. The highest electron density is shown on HOMO -1, which implies that is more favoured as a site by electrophilic attack. Therefore, the monoalkene formed in such a way shown below.
The monocarbene has a higher energy, which may result from the torsion and strain due to the formation of the new bonding.
Part 2
Optisimised geometries and IR spectra are obtained after the submission of the gaussian file to SCAN.
| Molecules | C=C stretch
(cm-1) |
C=C stretch
(cm-1) |
C-Cl stretch
(cm-1) |
out of plane C-H stretch
(cm-1) |
|---|---|---|---|---|
| Molecule 12 | 1757 | 1736 | 770 | 686 |
| Monocarbene | 1757 | n/a | 805 | 672 |
 |
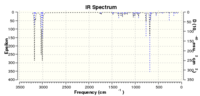 |
The peaks of monocarbene are generally higher than that of molecule 12. This is due to the C=Cl σ* orbital and the exo π-orbital overlap, which stabilises the whole molecule.
Monosaccharide chemistry: glycosidation
During the glycosidation, two sugars could be obtained due to the neighbouring group participation from the adjecent acetyl group.
The reaction of glycosidation undergoes as follow:
 [16]
[16]
To keep the computational demand minimal, R group is taken as Me. The possible mechanism is shown in Figure 4.[17]
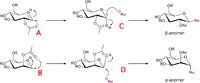
For each structure of A and B a conformer is drawn for each with the acyl group pointing above or below the plane of the cation, A' and B'. The structures are optimised using MM2 force field and MOPAC/PM6 method. The results are shown in the Table 10.
| Molecules | Stretch
(kcal/mol) |
Bend
(kcal/mol) |
Stretch-Bend
(kcal/mol) |
Torsion
(kcal/mol) |
Non-1,4 VDW
(kcal/mol) |
1,4 VDW
(kcal/mol) |
Charge/Dipole
(kcal/mol) |
Dipole/Dipole
(kcal/mol) |
Total Energy
(kcal/mol) |
|---|---|---|---|---|---|---|---|---|---|
| A | 2.4246 | 10.9185 | 0.9823 | 3.4172 | -1.3283 | 19.1468 | -12.0693 | 6.0114 | 29.5031 |
| A' | 2.100 | 9.9046 | 0.8832 | 1.2707 | -1.4837 | 18.8209 | -9.8806 | 5.5435 | 27.3685 |
| B | 1.9643 | 10.2073 | 0.7349 | 3.5485 | -2.3727 | 17.3133 | -4.5471 | 1.5533 | 28.401 |
| B' | 2.4417 | 11.2226 | 0.9808 | 3.4136 | -1.2465 | 19.2025 | -12.8412 | 6.0172 | 29.1907 |
| Intermediates | MM2 Force Fields (kcal/mol) | MMFF94 Force Fields (kcal/mol) |
|---|---|---|
| A | 29.5031 | -91.6541 |
| A' | 27.3685 | -88.72873 |
| B | 28.401 | -68.22976 |
| B' | 29.1907 | -91.64555 |
The results obtained indicates that B is more thermodynamically stable.
The same has applied to C and D by testing them together with the C' and D'. The results are shown below.
| Molecules | Stretch
(kcal/mol) |
Bend
(kcal/mol) |
Stretch-Bend
(kcal/mol) |
Torsion
(kcal/mol) |
Non-1,4 VDW
(kcal/mol) |
1,4 VDW
(kcal/mol) |
Charge/Dipole
(kcal/mol) |
Dipole/Dipole
(kcal/mol) |
Total Energy
(kcal/mol) |
|---|---|---|---|---|---|---|---|---|---|
| C | 2.0447 | 13.8824 | 0.7314 | 9.8701 | -2.6194 | 17.9702 | -10.2509 | -2.2249 | 29.4036 |
| C' | 2.7918 | 18.0285 | 0.8580 | 9.4775 | -2.3725 | 19.1867 | 5.5435 | -1.6052 | 48.9186 |
| D | 1.9643 | 10.2073 | 0.7349 | 3.5485 | -2.3727 | 17.3133 | -4.5471 | 1.5533 | 28.401 |
| D' | 2.4417 | 11.2226 | 0.9808 | 3.4136 | -1.2465 | 19.2025 | -12.8412 | 6.0172 | 29.1907 |
| Intermediates | MM2 Force Fields (kcal/mol) | MMFF94 Force Fields (kcal/mol) |
|---|---|---|
| C | 29.4036 | -91.6465 |
| C' | 48.9186 | -66.8742 |
| D | 45.4998 | -85.9359 |
| D' | 43.6590 | -91.66175 |
Structure of A, B C, and D shown as Jmol files after energy optimisation.
 |
 |
 |
 |
 |
 |
 |
 |
Structure based MINI project using DFT-based Molecular orbital methods
The total synthesis of (-)-Cubebol
Cubebol was first discovered by the flavour company, Firmenich in 2001.[19] It is also known as FEMA 4497, its structure shown below together with the Jmol file. It can be prepared from (-)- menthone, following a series of reaction forming cubebol.[20]

Following the reaction scheme, several molecules are tested and compared with the literature. The molecules are optimised and their IR and NMR spectra are predicted. [21]
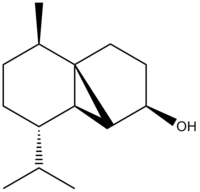 |
 |
 |
| Species | predicted values(cm-1) | literature values (cm-1) | %Differences |
|---|---|---|---|
| O-H stretch | 3168 | 3350 | -5.4% |
| C-H stretch(nujol)(sym) | 2992 | 2951 | 1.39% |
| C-H stretch(nujol)(asym) | n/a | 2860 | |
| C-O stretch | 1520 | 1490 | 2.01% |
| CH2, CH3 deformation | 1144 | 1142 | 0.18% |
| =C-H, =CH2 out of plane bending | 968 | 910 | 6.37% |
 |
 |
 |
| Species | predicted values(cm-1) | literature values (cm-1) | Differences(cm-1) |
|---|---|---|---|
| C-H stretch | 3038 | 2961 | 2.6% |
| C-H stretch(nujol)(sym) | n/a | 2931 | |
| C-H stretch(nujol)(asym) | n/a | 2872 | |
| C=O stretch | 1806 | 1712 | 5.5% |
| C-H out of plane bend | 1512 | 1455 | 0.18% |
| =C-H, =CH2 | 1302 | 1369 | 6.37% |
| =C-H, =CH2 | n/a | 1315 | 3.9% |
| CH rocking | 777 | 750 | 6.37% |
 |
 |
 |
| Species | predicted values(cm-1) | literature values (cm-1) | Differences(cm-1) |
|---|---|---|---|
| C-H stretch (nujol)(sym) | 2989 | 2955 | 0.91% |
| C-H stretch(nujol)(asym) | 2982 | 2929 | 1.81% |
| C-H stretch | 2905 | 2851 | 1.89% |
| CO stretch | 1456 | 1463 | 0.48% |
| C-H out of plane bend | 1512 | 1367 | 0.18% |
| =C-H, =CH2 stretch | 910 | 918 | 6.37% |
| =C-H, =CH2 out of plane bending | 875 | 836 | 3.9% |
 |
 |
 |
| Species | predicted values(cm-1) | literature values (cm-1) | Differences(cm-1) |
|---|---|---|---|
| OH stretch | n/a | 3390 | |
| C-H stretch(nujol)(sym) | 2976 | 2956 | 0.68% |
| n/a | 2935 | ||
| C-H stretch(nujol)(asym) | n/a | 2862 | |
| C-O stretch | 1416 | 1447 | -2.14% |
| =C-H, =CH2 stretch | 1360 | 1368 | 0.58% |
| =C-H, =CH2 stretch | 1328 | 1320 | 0.60% |
| CH2,CH3 deformation | 1192 | 1193 | 0.08% |
| CH2,CH3 deformation | 1144 | 1151 | 0.61% |
| =C-H, =CH2 out of plane bending | 944 | 946 | -0.21% |
| =C-H, =CH2 out of plane bending | 936 | 931 | 0.54%
|
The molecular modelling techniques applied here generate reasonably good IR spectra as indicated by the difference percentage. However, the NMR obtained from the prediction is so much higher than the literature values. The results of the NMR spctra are attached at the end as txt files.
References
- ↑ Cycloaddition of Cyclopentadiene DOI:http://commons.wikimedia.org/wiki/File%3ACyclopentadiene_dimerisation.svg
- ↑ H. Rzepa, 2nd Year Conformation Analysis Lecture Notes,Imperial College London, 2011 DOI:http://www.ch.ic.ac.uk/local/organic/pericyclic/
- ↑ J. Clayden, N. Greeves, S. Warren and P. Wothers, Organic Chemistry, Oxford University Express, New York, USA, 2001, pp916-917 ISBN:978-0-19-850346-0
- ↑ J. E. Jones, Proceedings of the Royal Society of London. Series A, 1924, 106, 463-477 DOI:10.1098/rspa.1924.0082
- ↑ M. E. Jamróz, S. Gałka and J. C. Dobrowolski, Journal of Molecular Structure: THEOCHEM, 2003, 634, 225-233 DOI:10.1016/S0166-1280(03)00348-8
- ↑ DOI:http://en.wikipedia.org/wiki/Molecular_vibration
- ↑ S. E. Barrows and T. H. Eberlein, Journal of Chemical Education, 2005, 82, 9, 1329 DOI:http://pubs.acs.org/doi/abs/10.1021/ed082p1329#citing
- ↑ DOI:{{{1}}}
- ↑ DOI:http://mason.gmu.edu/~aphan6/projects/protein/PART%20III.htm
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319 DOI:http://dx.doi.org/10.1016/S0040-4039(00)92617-0
- ↑ W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891. DOI:http://dx.doi.org/10.1021/ja00398a003
- ↑ A.B. McEwen, P. v. R. Schleyer, J. Am. Chem. Soc., 1986, 108, 14, pp 3951-3960 DOI:10.1021/ja00274a016
- ↑ W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891. DOI:http://dx.doi.org/10.1021/ja00398a003
- ↑ DOI:http://dx.doi.org/10.1039/P29920000447
- ↑ http://symmetry.otterbein.edu/common/images/flowchart.pdf
- ↑ https://wiki.ch.ic.ac.uk/wiki/index.php?title=Mod:organic#Monosaccharide_chemistry:_glycosidation
- ↑ https://wiki.ch.ic.ac.uk/wiki/index.php?title=Mod:organic#Monosaccharide_chemistry:_glycosidation
- ↑ https://wiki.ch.ic.ac.uk/wiki/index.php?title=Mod:organic#Monosaccharide_chemistry:_glycosidation
- ↑ J. C. Leffingwell, Cool without Menthol & Cooler than Menthol and Cooling Compounds as Insect Repellents DOI:http://www.leffingwell.com/cooler_than_menthol.htm
- ↑ K L. McPhail et al, Bioorganic & Medicinal Chemistry, 2011, 19, 6675–6701., DOI:10.1016/j.bmc.2011.06.011
- ↑ D.M. Hodgsont, S. Salik and D. J. Fox, J. Org. Chem., 2010, 75, 7, pp2157-2168 DOI:http://pubs.acs.org/doi/pdfplus/10.1021/jo9022974