Rep:Mod:hb1013
Computational Chemistry: Transition States and reactivity
Introduction
By using mathematical method and chemistry theories, reaction of molecules can be simulated by computer. In this module, geometry and reactivity of cycloaddition were investigated. Transition states, molecular orbitals of three different types of cycloaddition were explored by Gaussian and GaussView.
Potential energy surface(PES) describes the energy of a molecule as a function of its geometry. The first derivative of energy is zero, the second derivative energy is negative and the transition state is the highest position in the pathway(saddle point). Two methods can be used to find out the transition state: frequency calculation and Intrinsic Reaction Coordination(IRC).
IRC is a method that can check whether transition state is in the position of the highest energy by exploring energy changing in both directions from the transition state.
Also, in this module optimisation of the molecules is really important to get more accurate geometry and energy of the transition state due to it can determine the geometry with lowest energy.
Nf710 (talk) 12:16, 7 April 2017 (BST) your definition of the TS is incorrect. It has positive curvature in every coridate apart from one one which is the reaction coordinate. hence saddle point
Exercise 1: Reaction of Butadiene with Ethylene
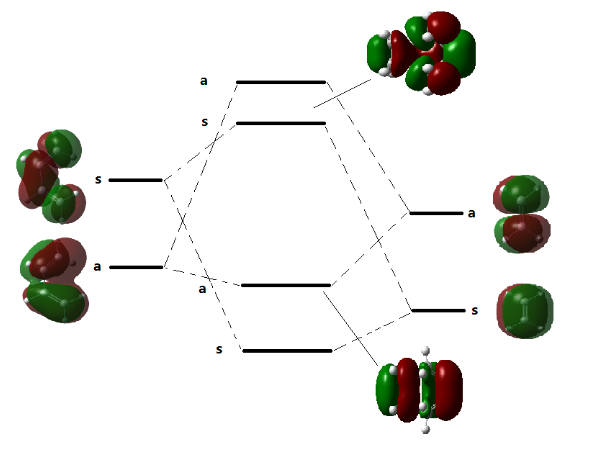
(Fv611 (talk) 16:00, 5 April 2017 (BST) You know that the TS HOMO is symmetric from the picture you have on there, so why label it as antisymmetric? The TS MO you are showing at the top is the LUMO, but that is not clear from your diagram. Additionally, you are missing two of the TS MOs, and the electron occupancy. The diagram would also have been a lot more clear if you had drawn schematics of the FOs and of their interactions.)
Fragments Orbitals(FOs) with the same symmetry can interact and overlap with each other. In Fig.1, transitions states are formed by overlap of HOMO and LUMO of FOs with the same symmetry.
(Fv611 (talk) 16:00, 5 April 2017 (BST) You are missing the discussion on orbital overlap.)
The Cis-butadiene and ethylene were drawn in GaussView and optimised at PM6 level.
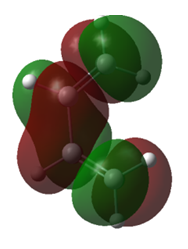
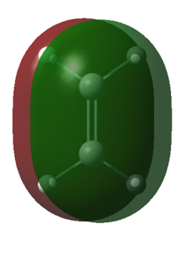
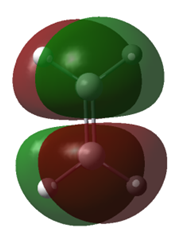
| Molecule | Cis-butadiene | Cis-butadiene | Ethene | Ethene |
| MO | HOMO | LUMO | HOMO | LUMO |

|
|
|
| |
| Symmetry | Anti-symmetric | Symmetric | Symmetric | Anti-symmetric |
| Energy/a.u. | -0.35168 | 0.01103 | -0.39228 | 0.04256 |
Table 1. Homo and LUMO of Cis-butadiene and ethene.
Optimised transition state is shown below.

To confirm this transition state is correct, frequency calculation and IRC are shown below.
(You also need a gradient of zero to be a TS Tam10 (talk) 17:33, 3 April 2017 (BST))
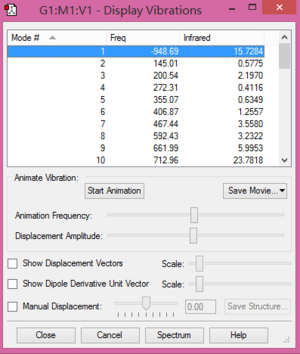

As shown in Figure 3 and Figure 4, there is only one negative frequency for the transition state and a saddle point, which means this structure is correct.
(Fv611 (talk) 16:00, 5 April 2017 (BST) You didn't show the vibration corresponding to the bond formation, nor indicated whether this event is synchronous or asynchronous.)
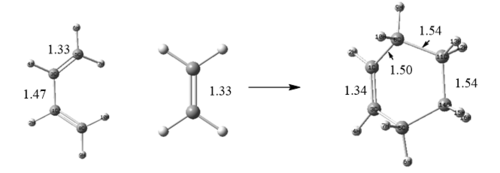
According to Fig. 2 and Fig. 5, Bond lengths of reactants, ts and product can be known.
| Bond | Length in reactant | Length in TS | Length in product |
| C=C in TS of cis-Butadiene | 1.33 | 1.38 | 1.50 |
| C-C in TS of cis-Butadiene | 1.47 | 1.41 | 1.34 |
| C=C in TS of Ethene | 1.33 | 1.38 | 1.54 |
| Partially formed bond in TS | n/a | 2.11 | 1.54 |
Table 2. Bond lengths of reactants, ts and product. Units in Å.
(Fv611 (talk) 16:00, 5 April 2017 (BST) Labelling is very confusing. If you optimised the reactants to a TS, you shouldn't have, as the reactants won't have a transition state.)
Normally, C-C single bond is 1.54 Å and C=C double bond is 1.33 Å.[1] But in the transition state C-C and C=C are 1.41 Å and 1.31 Å respectively, which means C-C single bond is shortened and C=C double bond is elongated. The partially formed bond in TS is shortened to 2.11 Å and it is shorter than 2 * Van der Waal's radius of carbon (1.7Å).[2] This indicates overlap of p-orbitals between terminal carbons in cis-Butadiene and ethene.
6-pi electrons in TS and according to Hoffmann rules, the reaction will be thermally allowed when p-orbitals interaction between terminal carbons in cis-Butadiene and ethene includes two types: superfacial and antarfacial.
| Molecule | TS cyclohexene | TS cyclohexene |
| MO | HOMO | LUMO |
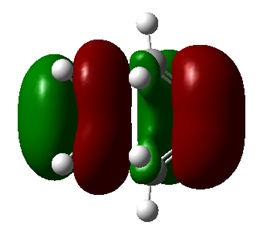
|
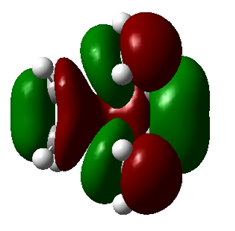
| |
| Symmetry | Symmetric | Symmetric |
| Energy a.u. | -0.32533 | 0.01732 |
Table 3. TS of HOMO and LUMO.
(Fv611 (talk) 16:00, 5 April 2017 (BST) You are missing the antisymmetric TS MOs)
Cis-Butadiene has one AS HOMO and one S LUMO and ethene has one S HOMO and one AS LUMO. Therefore, there are two possible interactions in this reaction. Because the diene is nucloephilic and the dienophile is electrophilic, the interaction between AS HOMO of cis-butadiene and AS LUMO of ethene is more favoured.
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
| Cyclohexdiene | 1,3-Dioxole | |
|
| |
| endo | exo | |
| TS | 
|

|
| product | 
|
|
Table 4. Structures of optimised reagents, TS and products.
(Endo TS looks wrong from this angle Tam10 (talk) 17:33, 3 April 2017 (BST))
| Orientation | endo | endo | exo | exo |
| MO | HOMO | LUMO | HOMO | LUMO |

|
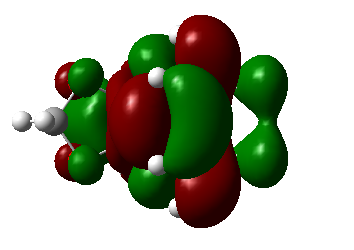
|

|
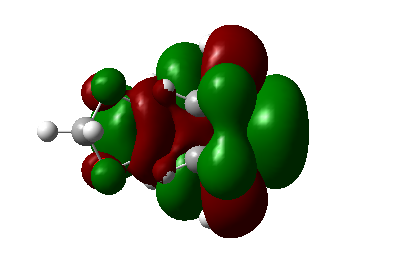
| |
| Symmetry | As | As | As | As |
(These are symmetric, not antisymmetric! You need to have an MO diagram for this exercise Tam10 (talk) 17:33, 3 April 2017 (BST))
Table 5. MOs of TS of endo and exo orientations(secondary orbital overlap in the endo).
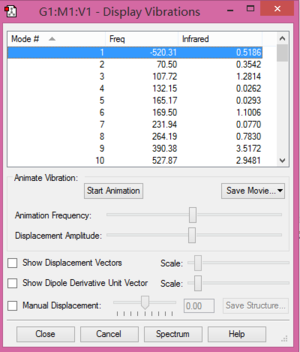
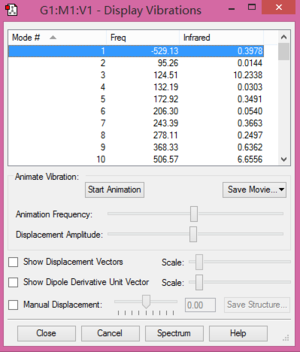
Fig.6 and Fig.7 ensure the endo and exo structures are correct.

This reaction is a normal demand DA reaction, the reason is C=C double bond is more electron deficient due to existing of two electron withdrawing oxygens in the dienophile.
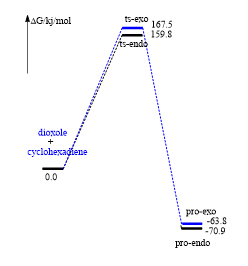
In this reaction, product of endo is thermodynamic product due to lower energy makes it more stable, and secondary orbital interaction in endo can provides an extra interaction in the reactants, it causes lower energy barrier of endo product.Therefore,product of endo is kinetically favoured as well.
Nf710 (talk) 12:19, 7 April 2017 (BST) You have got the correct energies and therefore the correct TS however you have not give a good argument as to why you have got the conclusions you have got
Exercise 3: Diels-Alder vs Cheletropic
| Exo D-A Reaction | Endo D-A Reaction | Cheletropic Reaction | |
| Structure | 
|
|
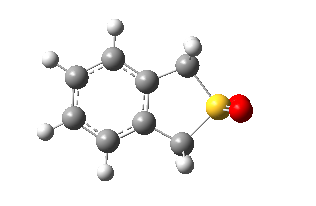
|
| IRC | 
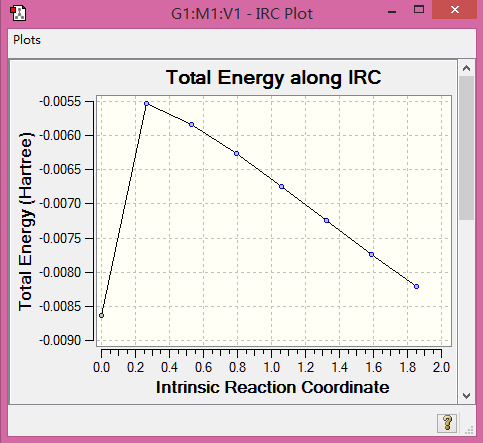
|
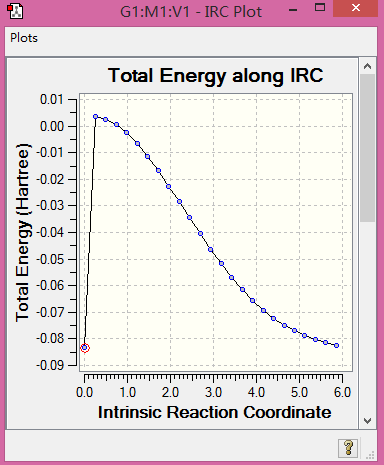
|
|
(IRCs are incomplete. They probably crashed on the corrector steps. We include a section on what to do when this happens, or you could have asked a demonstrator Tam10 (talk) 17:33, 3 April 2017 (BST))
Table 8. Structures and IRC.
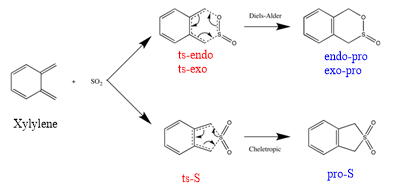
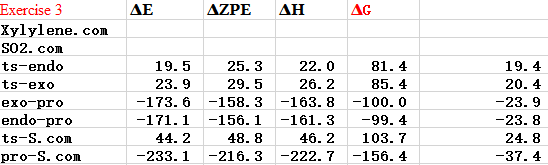
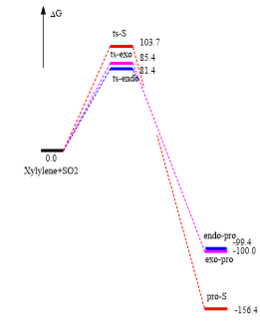
(Figure 10 is unnecessary. You shouldn't use fictitious curved lines anyway Tam10 (talk) 17:33, 3 April 2017 (BST))
This reaction is exothermic so has negative E and because of high free energy of xylyene, so it is very unstable. The formation of endo one is kinetically favoured, cheletropic one is thermodynamically favoured if there is enough energy. Futhermore, six-membered ring product like bezene to acheieve resonance form that can form an electron pi cloud and make the electrons delocalised.
Conclusion
The lengths of carbon bonds will change in D-A reactions, and will be shorter than VDW's radius. Same symmetry Orbitals can overlap with each other and have none 0 orbital overlap integrals.
Product of endo is thermodynamic product due to lower energy makes it more stable, and secondary orbital interaction in endo can provides an extra interaction in the reactants so product of endo is also kinetically favoured.
TS has the maximum energy and the the reaction of sulfur dioxide and o-Xylylene cycloaddition is exothermic and because of high free energy of xylyene, so it is very unstable.
