Rep:Mod:hardi180288
Imperial College
London
MODULE 1
Note: This page is optimized for a window of width ~1400px
|
|
Modelling using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
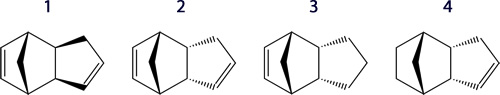
The cyclodimerisation of cyclopentadiene is a π 4 s + π 2 s Diels-Alder cycloaddition, which proceeds under thermal conditions proceeds via Hückel:
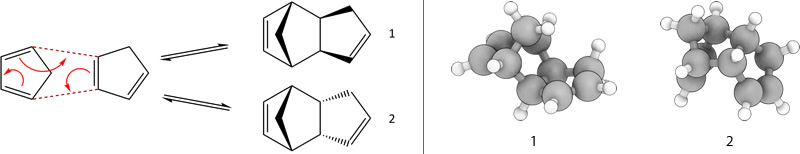
The Exo dimer 1 has a total energy of 31.8764 kcal/mol, whilst the Endo dimer 2 has a total energy of 33.997 kcal/mol. Thus from a thermodynamic perspective, the exo conformation is less strained so is preferred.
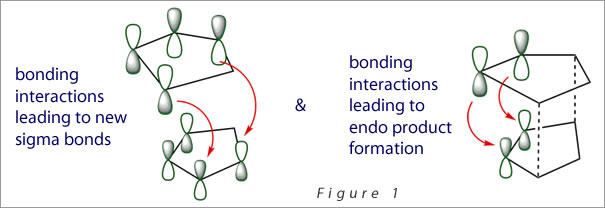
However, the endo product predominates which can be explained by considering secondary orbital interactions.[1] As the frontier orbitals approach each other in the reaction, they are of the correct symmetry for bond formation. If one then considers the situation at the back of the diene, the symmetry of the orbitals is again correct for bond formation. Although this favourable interaction does not lead to the formation of any new bonds, it does influence the stereochemistry by resulting in the formation of the endo product (Figure 1).
|
The dihydro derivative 3 has a total energy of 35.9266 kcal/mol, whilst that of 4 is 31.1520 kcal/mol. From this it can be seen that derivative 4 is more thermodynamically stable, thus it is thermodynamically easier to hydrogenate the double bond endo to the bridge.
Since the stretching, torsion and van der Waals and values are of notable similarity for the two molecules, the primary difference is from the bending. This can be seen in observing the 3 and 4 sp2 carbon bond angles. In the favoured conformation (4) the angles are 112.4° and 113.0° on each side of the sp2 carbon, whilst in the less stable conformation (3), these angles are further from the ideal 120° with both being 107.6°.
| 1 | 2 | 3 | 4 | ||||||||||||
|
|
|
|
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+) analogue
6 is formed as a result of chelation control.[2] The high regio- and stereoselection in the additon of the Grignard is due to coordination between the Grignard reagent and the amide oxygen, as shown by (a). It is conformationally most stable to have the amide carbonyl group above the plane of the pyridine ring, as shown in (b). Thus the intramolecular delivery of the methyl group via a six centered transition state can only proceed to give alkylation of the pyridine ring, with the absolute stereochemistry of (b).
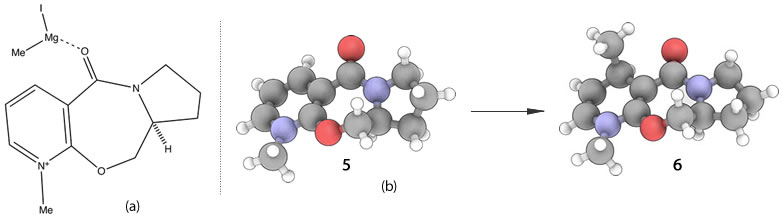
It is evident from the stereochemistry of the product 8 and the 3D structures of both 7 and 8 that the aniline reacts on the opposite face with respect to the carbonyl of the lactam. Consequently it can be deduced that the diasterofacial selectivity is indeed due to the steric control exerted by this carbonyl interfering with the incoming aniline. The fundamental origin of the lactam carbonyl group orientation is likely due to the presence of the methyl and isopropyl groups on the lactam itself. The unsubstituted adjoining phenyl constrains the geometry, such that the methyl must adopt an axial orientation to minimize interactions with the bulky isopropyl group. Thence the sp2 carbonyl group is angled down relative to the aforementioned methyl.

NOTE: Including the Grignard reagent in the MM2 calculation simply causes the calculation to fail.
|
Using the MM2 force field, the energy of 5 was optimized to a relatively low energy which was only reduced slightly after a number of manipulations (particularly the O-CH2 bond). By contrast, the first energy optimization of 8 resulted in both the carbonyl and the aniline pointing up. This is sterically and geometrically disfavoured as demonstrated by the obscenely high energy of this conformation (832.592kJ mol-1, Note: C-C bond strength ~348kJ mol-1). Further manipulations and MM2 force field optimizations with the carbonyl group pointing anti to the aniline then gave a substantially reduced energy. An attempt was made to find a conformational energy minimum for 7 in which the carbonyl group similarly pointed out of the plane of the lactam (in a similar direction to the methyl of said lactam). However, as can be seen when considering the 3D position of atoms, this resulted in too large a steric interaction between the methyl and the carbonyl to obtain any quantitative information.
Evidently molecular mechanics is poor at modelling the interaction of metal ions and the stereochemical influence they may exert. In addition, stereoelectronic effects (particularly in any transition states, which themselves are totally ignored by molecular mechanics) need to be considered when modelling the product of reactions such as that of nucleophilic additon to the pyridinium ring. Thus a quantum mechanical approach would be more suitable, in which specific electrons and the full molecular orbitals of the molecules must be considered.
| 5 | 6 | 7 | 8 | ||||||||||||
|
|
|
|
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Isomer 10
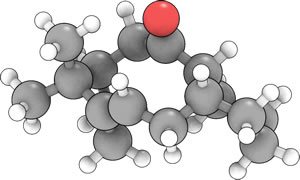 |
Using molecular mechanics, isomer 10 was initially optimized and thought to have an energy of 54.5438kcal mol-1, with the cyclohexane group adopting a twist-boat conformation. After successive manipulations the final (and lowest energy) conformation was realised with an energy of 48.8749kcal mol-1, in which the said cyclohexane group adopts the more stable chair conformation. It should also be noted that a higher energy conformation exists with the alkene bond pointing more up (with the carbonyl) and the bridge closer in to the main part of the molecule. This conformation is noteworthy since it is of relative low energy. A table summarising some of these successive optimisatons has also been included:
|
This conformation is evidently lowest in energy due to the reduced steric interaction between the carbonyl and the cyclohexane group. In addition, this conformation also reduces steric interaction of the bridging element with this carbonyl.
Isomer 11
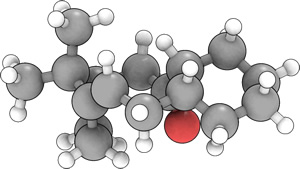 |
The energy of 11 was optimized in a similar fashion to 10, using molecular modelling within the ChemBio3D application. One of the initial conformational minima obtained during the optimization process had the carbonyl pointing down but clearly angled out, away from the cyclononene ring. The adjoining cyclohexane group was also seen to adopt a twist-boat conformation. The combination of these geometrically disfavoured elements produced a total (proposterous) energy of 128.6410kcal mol-1. Manual manipulations to improve the geometry of the cyclononene ring gave a final optimized energy of 54.4786kcal mol-1. However, in this conformation, the cyclohexane again adopted a twist-boat conformation. Although probable in a molecule with large substituents sterically forcing the geometry of the nonene ring [3], such elements are not present in this molecule. Hence it was thought that the cyclohexane, in the more stable chair conformation, would reduce the overall energy of the molecule. This was indeed the case, with a final energy of 11 after the optimization procedure described above of 44.2651kcal mol-1. These energies are summarised in the table below.
|
The alkene reacts abnormally slowly since it is a hyperstable alkene. As reported in the original paper[4] these alkenes have less strain than the parent hydrocarbon and have a negative "OS" (olefin strain) value. These alkenes are unreactive due to the extra stability from their cage structure, which is less strained than the parent polycycloalkane, rather than from steric hindrance or enhanced π bond strength.
| 10 | 11 | ||||||
|
|
How one might induce room temperature hydrolysis of a peptide
Isomer 13
 |
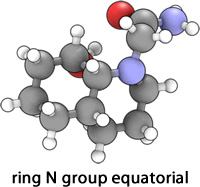 |
A model of isomer 13 was built, initially with the ring N-substituent axial. Using a similar optimization strategy (ChemBio3D MM2) as previously employed, an optimal energy of 18.8742 kcal mol-1 was obtained. It should be noted that this conformation of lowest energy must have both cyclohexane rings adopting chair conformations, however the stereochemistry of the hydrogens on the two carbons joining the rings means that this cis-decalin conformation will be inherently of higher energy than isomer 14.
Similarly, a model of 13 with the ring N-substituent equatorial was constructed. This conformation is expected to be of lower energy than with the ring N-substituent axial, since it is typically more stable to have a (large) group equatorial rather than axial due to 1,3-Diaxial compression. This was indeed found to be the case, with an optimized energy of 15.4533 kcal mol-1 obtained. On viewing the 3D structure for this conformation, it is evident that a further reduction in energy is due to the presence of a H-bond between the carbonyl oxygen and the hydroxy group hydrogen.
Isomer 14
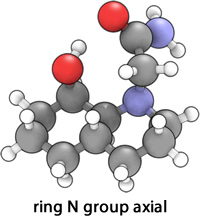 |
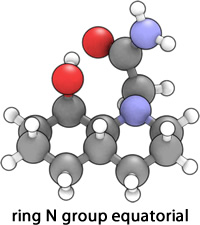 |
It was expected that isomer 14 would be of an overall lower energy for both conformations, with both the N group axial and equatorial relative to 13, since the two cyclohexane rings are of optimal geometry; the isomer adopts a trans-decalin structure with an all-chair conformation that has every bond staggered from every other bond[5]. A direct consequence of this is that a H-bond similar to that of the equatorially substituted 13 is present in both axial and equatorially substituted conformations of 14, again resulting in a lowering of the energy. Thus it is expected that the equatorially substituted conformation will be of lower energy simply due to the lowering of 1,3 Diaxial compression. This prediction was indeed realised, as the isomer of 14 with the N ring substituent axial had an optimized energy of 11.9533 kcal mol-1, whilst that of the equatorial conformer was 9.6501 kcal mol-1.
It can also be noted that there is a greater energy difference between the axial and equatorial conformations for 13 when compared to 14. [6]As shown by the reaction schemes below, when 13 is in the equatorial position, the ethylamido group undergoes intramolecular nucleophilic attack by the 8-hydroxyl group. Alternatively, in 14, the ethylamido group must first (at energetic cost) convert to the axial position.
 |
Thus isomer 13 reacts 40x faster.
In neutral solution at 25°C, peptide bonds take about 500 years to hydrolyse for the C-terminal bond of acetylglycylglycine. This represents uncatalysed attack by water on the peptide bond, since it is insensitive to changing either pH or ionic strength. Thus no intramolecular nucleophilic attack can occur. Alternatively, for the equivalent enzyme reactions, a comparison of rate constants shows a very strong binding of the altered substrate in the transition states, which provides the basis for the increase in reaction rate.[7]
| 13 N group axial | 13 N group equat. | 14 N group axial | 14 N group equat. | ||||||||||||
|
|
|
|
Modelling Using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
Part 1
|
 |
The molecular orbitals and energies are of notable similarity for the two molecular orbitals methods, suggesting that the geometry of 12 can be modelled quite well using a relatively basic quantum mechanical treatment. Since the energies obtained using different force fields cannot be compared, the energy of the molecule (at the HOMO level) unfortunately cannot be directly compared with the energy of the molecule gleaned from the simple molecular mechanics approach.
The primary difference between the classical and quantum mechanical approaches is in the reactivity of the molecule. [8]The diene reacts with electrophilic agents (like dichlorocarbene) regioselectively on the double bond endo to the chlorine substituent, opposite the cyclopropyl ring. This is due to (antiperiplanar) stabilising interactions between the Cl-C σ* orbital and the occupied exo π orbital. This means that the endo double bond is more nucleophilic both from a frontier orbital and an electrostatic perspective. From this, it is predicted that there should be a geometrical distortion of the exo double bond towards the bridgehead carbon, which was indeed (via molecular modelling) observed to be the case, demonstrated by the image to the right.
Part 2
|
|||||||||||||||
|
||||||||||||
It is expected that the stabilizing interaction of the Cl-C σ* orbital with the (occupied) exo π orbital will causing a weakening of the C-Cl bond as there is effectively more electron density present in an anti-bonding orbital. Concurrently, one would expect a reduction in bond order for the exo C=C bond, relative to the endo C=C bond. This is indeed the case as in the diene, the exo C=C bond is weaker/ has a lower vibrational frequency (1740.75 cm-1 vs 1760.91 cm-1) . Furthermore, one would expected the exo-hydrogenated molecule to have a (relatively) stronger C-Cl bond with a higher vibrational frequency due to the loss of the Cl-C σ* / exo π orbital overlap. Again this was observed, with C-Cl vibrational frequencies of 772.634 cm-1 and 776.989 cm-1 for the diene and monohydrogenated product respectively.
Structure based Mini project using DFT-based Molecular orbital methods
Assigning regioisomers in "Click Chemistry"
This mini-project involves an investigation into the 1,3-diploar cycloaddition between an azide and an alkyne giving a 1,2,3-triazole, via the use of both Cu(I) and Ru(II) catalysts proceeding via a facile "click reaction" to give different isomers.
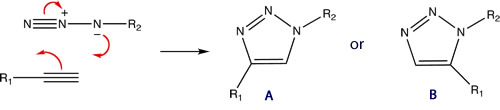
The two 3D diagrams below have been labelled such that the individual atoms can be referenced concisely
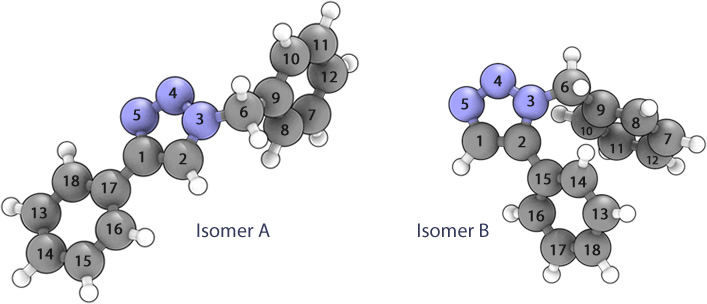
Isomers A and B could be differentiated spectroscopically by both 13C NMR (and possibly by 1H depending on the resolution of the instrument).
Isomer A
By 13C NMR a number of spectroscopic features would be observed:
A high field peak of relative intensity 1 would be observed due to carbon 6 which is free from any aromatic system. The next peak (moving downfield) to be observed, again of relative intensity 1, would be due to carbon 2 which is the unsubstituted carbon of the triazole system. This carbon is expected to present itself in this position due to the weaker aromaticity of the triazole system relative to the phenyl groups along with the more electropositive adjoining hydrogen, causing a slight shift upfield.
Again moving downfield, the next peak to be observed would be a singlet of relative intensity 2, due to carbons 16 and 18. This is expected since carbons 1 and 17 are participants in a doubly aromatic system of the phenyl-triazole system. As a consequence there is a distortion of the aromatic system towards the triazole, thus carbons 16 and 18 are less deshielded, so appear further up field.
The next peak would likely be in fact a number of closely spaced peaks of overall integration 8, due to carbons 7, 8 and 10-15 of the two aromatic phenyl groups. Following this would be a peak due to carbon 17, as a consequence of the proposed perturbation of the π system, it would be in a less deshielded environment than carbon 9 (which would be the next observed peak downfield). Both of which, however, are further downfield than the other carbons of the phenyl groups since they do not have (more electropositive) hydrogen atoms shielding them. Finally, considerably further upfield would be a peak due to carbon 1 which is fully engaged in two aromatic systems, thus is highly deshielded.
Calculated 13C NMR Spectrum
|
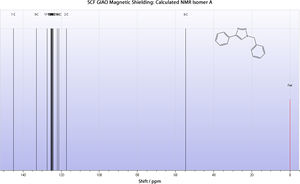
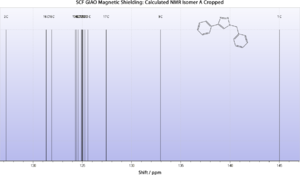

From the above it is evident that for isomer A at least, the calculated spectrum agrees remarkably well with the literature. Thus it is likely that their geometrical assignment was accurate.
Isomer B
Predicting a 13C spectra for isomer B, it would have the following spectroscopic features:
Moving downfield, the first peak to be observed would again be the carbon 6 of the methylene group which is free from an aromatic system, with two (relatively electropositive) adjoining hydrogen atoms. Following this, a series of overlapping peaks/ a multiplet would be observed of overall integration 11, due to carbons 7, 8 and 10-18 of the two phenyl systems. It should be noted that the twelfth phenyl carbon is carbon 9, which is observed further upfield due to the standard perturbation free π system of the phenyl group which deshields it.
Possibly due to a favourable interaction between the two π systems of the phenyl groups (which can come into much closer proximity of one another in isomer B), the phenyl group attached to carbon 2 may be more electron-withdrawing, thus carbon 1 is observed further downfield and suffers greater deshielding than 'standard' C-H carbons of the phenyl groups.
Finally (and similarly to isomer A), the carbon of the triazole to which the phenyl group is directly atttached (carbon 2 in this case) will be observed furthest downfield since it is very strongly deshielded by the two conjugated π systems of the aforementioned triazole and phenyl groups.
Calculated 13C NMR Spectrum
|
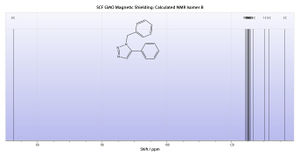
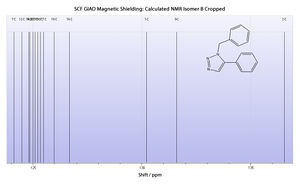
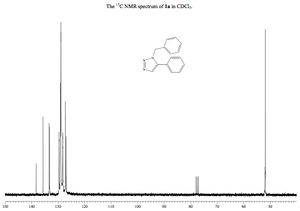
From this it is demonstrably clear that the literature NMR correlates exceptionally well to the calculated NMR. Not only does this verify the findings in the literature, confirming the classification of the isomer in question, but also proves the usefulness and accuracy of the NMR modelling method.
Mechanism of the reaction and the selectivity of one product isomer
The general reaction scheme for this type of reaction has been given at the start of this topic, however, the selectivity of the reaction is influenced by the catalyst used, as shown below[11]:
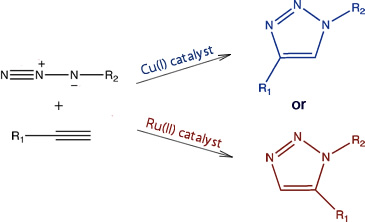
These selectivities can be understood in terms of the catalytic cycles of the two processes:
Catalytic cycle for Cu(I) catalysts giving 1,4 substituted triazoles (adapted from[12]):
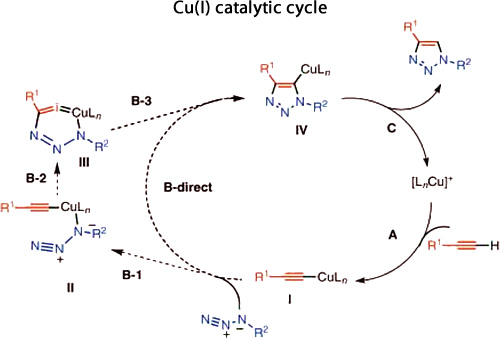
Alternatively, the catalytic cycle for the Ru(II) catalysed process to give 1,5 disubstituted triazoles is less well known. It has been suggested[13] that there is an oxidative coupling of an alkyne and an azide on the ruthenium, initially giving a six-membered ruthenacycle (with A being more likely than B in the scheme below). This then undergoes a reductive elimination to give the triazole product.
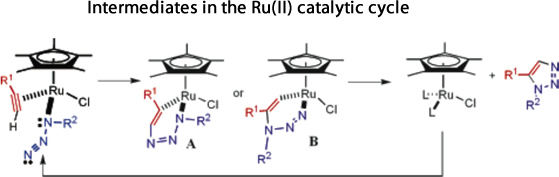
Molecular mechanics most certainly cannot be used to model this process since it is entirely influenced by the catalysed intermediates, which would be neglected. A molecular orbital based calculation method would be feasible, but a (good) proposed catalytic sequence would need to be obtained first, before the relative energies and orbital interactions of each step could be computed and their likelihood assessed.
Acknowledgements / Comments
All images, unless otherwise referenced, have been produced myself using a combination of ChemBio3D, Gaussview, Microsoft Paint, ChemDraw Ultra, Adobe Fireworks CS4 and QuteMol. Although in the public domain, it is humbly requested that their use/reproduction be done so via contact (email:rob@gynx.org).
References
- ↑ Clayden et al., Organic Chemistry, 2001, p916.
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ Wilhelm F. Maier, and Paul Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103 (8), 1891-1900 DOI:10.1021/ja00398a003 )
- ↑ Clayden et al., Organic Chemistry, 2001, p863.
- ↑ M. Fernandes, F. Fache, M. Rosen, P.-L. Nguyen, and D. E. Hansen, 'Rapid Cleavage of Unactivated, Unstrained Amide Bonds at Neutral pH', J. Org. Chem., 2008, 73, 6413–6416 ASAP: DOI:10.1021/jo800706y
- ↑ Radzicka, A.; Wolfenden, R. J. Am. Chem. Soc. 1996, 118, 6105–6109: DOI:10.1021/ja954077c
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
- ↑ J. Am. Chem. Soc. 2005, 127, 15998, Supporting Information; DOI:10.1021/ja054114s )
- ↑ J. Am. Chem. Soc. 2005, 127, 15998, Supporting Information; DOI:10.1021/ja054114s )
- ↑ J. Am. Chem. Soc. 2005, 127, 15998; DOI:10.1021/ja054114s )
- ↑ Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596
- ↑ J. Am. Chem. Soc. 2005, 127, 15998; DOI:10.1021/ja054114s )