Rep:Mod:gzus
Module 1
Modelling Using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
 |
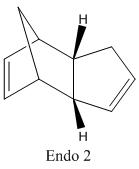 |
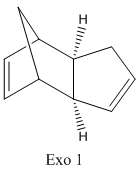
Chem3D was used to define both dimers of cyclopentadiene and their geometries were optimised using MM2 force field. The products of hydrogenation were compared, allowing a thermodynamic prediction of the relative ease of hydrogenation of each of the double bonds to be obtained. The relative energy contributions from the stretching (str), bending (bnd), torsion (tor), van der waals (vdw) and hydrogen bonding (H-bond) were analysed and used to determine the relative stability of both hydrogenation products.
Cyclopentadiene undergoes a Diel's Alder cycloaddition and dimerises to produce both exo and endo dimers. This mechanism involves 6 π electrons(4n+2, n=1) and thermally proceeds with Huckel topology involving suprafacial components. The formation of each dimer depends on the relative orientation of the reactants which determines whether the exo or endo adduct is formed. The endo adduct is found to be the major product and is confirmed by comparing the energies of the two isomers as can be seen in the table below. The exo isomer is lower in energy than the endo isomer and thus implies the reaction is kinetically controlled (irreversible conditions). Under thermodynamic control (reversible conditions) however, the more stable exo dimer would be expected to be the major product. The stereoselectivity for the product is governed by the endo addition rule and implies that the kinetic product possesses a transition state with the lowest energy and hence is formed the fastest. The transition state for the endo adduct is slightly more stable than that of the exo adduct and is confirmed by the comparison of the energies below.
 |
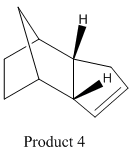 |
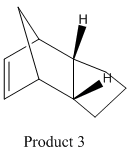
The endo dimer hydrogenates to one of the two dihydro derivatives 3 or 4. We can see that the calculated total energies for these molecules show that isomer 4 is lower in energy than 3.
Further analysis can be carried out on the total energy by looking at the relative contributions from stretching(str), torsion (tor), Van der Waals (vdw) and hydrogen bonding terms and can be seen in table 2. The contributions from the stretches, bends and torsions indicate strain in molecule and are higher in product 3 that 4. The bending energy has the largest contribution to the total energy in each case and is higher in 3 compared to 4. The torsion energy also has a significant contribution to the overall energy for both dimers although not as high as the bending energy. The reduction is bending energy of isomer 4 is accompanied by an increase in torsion energy. The non-1,4 VDW energy contributions are higher for 3 than 4 and are due to interactions between hydrogen atoms across the carbon rings. Therefore, product 3 is likely to be higher in energy as a result of the strain and destabilising hydrogen interactions present in the molecule.
| Molecule | Energy/kcalmol -1 |
|---|---|
| Exo 1 | 31.88 |
| Endo 2 | 34.98 |
| Dihydro Derivative 3 | 38.73 |
| Dihydro Derivative 4 | 29.25 |
| Mode | Product 3/kcalmol -1 | Product 4/kcalmol -1 |
|---|---|---|
| Stretch | 1.16 | 1.13 |
| Bend | 17.31 | 13.01 |
| Stretch-Bend | -0.70 | -0.56 |
| Torsion | 15.89 | 12.42 |
| Non-1,4 VDW | -1.88 | -1.33 |
| Dipole/Dipole | 0.16 | 0.14 |
In conclusion, product 4 would be expected to be formed as a result of thermodynamic hydrogenation. However, if the hydrogenation mechanism involves a late stage transition state then by invoking Hammond’s Postulate, the transition state for product 3 would be expected to be lower in energy than that of 4 and hence the energy of activation in forming 3 will be lower. Therefore, under kinetic control, product 3 could also be formed.
Sterochemistry of Nucleophilic Additions to a Pyridinium Ring (NAD+ analogue)
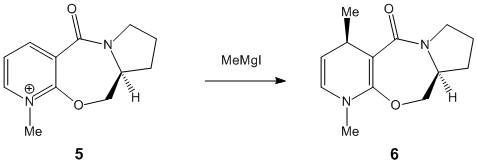
The optically active derivative of polinol (5) reacts with methyl magnesium iodide (Grignard reagent) to alkylate the pyridine ring in the 4-position with retention of stereochemistry shown in product (6). The selectivity of the reaction at the 4 position in the pyridinium ring produces a diastereoisomer with the methyl group antiperiplanar to the hydrogen atom of the stereocentre highlighted. This structure was proved using X-ray diffraction techniques by A. G. Schultz et al[1]. They found that the magnesium metal of the Grignard reagent coordinates to a lone pair on the carbonyl oxygen, forming a six membered transition state in which the methyl group adds above the plane of the pyridine ring at the 4 position.
 |
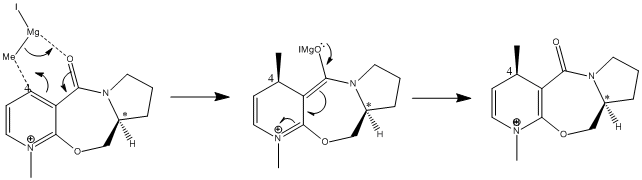 |
Upon minimising 5 using the MMFF94 force field calculation, the initial energy was found to be 57.49kcalmol-1 with a dihedral angle of -2o. This shows that the carbonyl is 2 degrees below the plane of the ring and is almost coplanar. Another optimisation was obtained using the MMFF94 force field calculation and led to a minimisation energy of 59.38kcalmol-1 with a dihedral angle of 37o. This corresponds to the carbonyl being positioned 37 degrees above the plane of the pyridine ring. Hence, when the magnesium metal coordinates to the carbonyl oxygen it directs the methyl group to the same face of the pyridine ring at position 4.
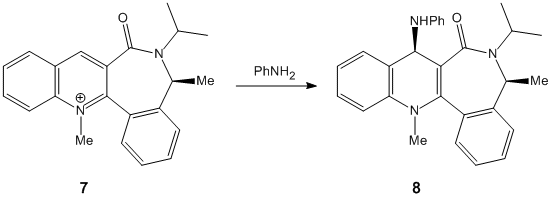
The pyridinium ring in 7 undergoes a stereoselective nucleophilic addition with the PhNH2 at the 4 position to give product 8 as can be seen from teh reaction scheme below. Optimizing with MFF994 gave an inital energy of 115.57kcalmol-1 with a dihedral of 37o and implies teh carbonyl group is above the plane of the ring.
 |
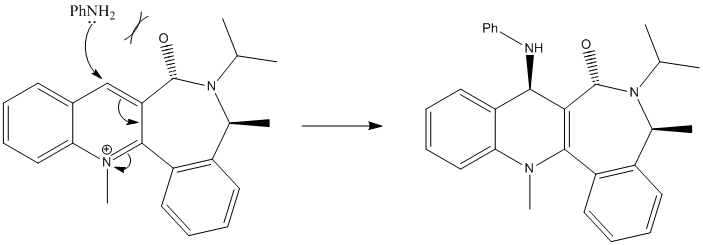 |
The amine group is sterically large and electron rich and therefore will repel the carbonyl oxygen as it approaches it. There is a large contributing steric factor which causes addition of the amine group to come from the opposite face of carbonyl group, resulting in a cis configuration to the lactam methyl group. The MeMgI component cannot be included because the program does not include the Magnesium atom type in the calculations as the metal-carbon bond cannot be distinguished. The MM2 force field does not take into account the aromaticity of the pyridine ring as the bonds cannot be related to simple diatomic molecules.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
 |
Both isomers (9) and (10) are key intermediates in the synthesis of Taxol. The carbonyl group may adopt the stereochemsitry of pointing upwards as seen in (9), or downwards as in (10). The total energy of both the isomers were obtained using the MM2 force field minimisation were found to be 46.39 and 38.79kcalmol-1 respectively. These correspond to the geometry of the six-membered ring seen in the structure, with the highest energy of 9 being due to the ring being in a twist boat conformation, whereas the significantly lower energy of 10 can be attributed to the adoption of a chair conformation.
This could be due to cycloalkenes are being more strained than their parent cycloalkanes as a result of increased transannular hydrogen interactions in the ring [2]. The strain energy for the cyclohexane in 10 is lower than that of 9, hence this particular alkene reacted slowly during the reaction due to its hyperstability. Electrophiles may not be able to approach the bridge-head alkene so readily due to steric hindrance from the methyl group above and the carbonyl bond below. This accounts for the lower energy seen for 10 from the MM2 minimisation.
However, when using the MMFF94 minimisation, the optimised energy values obtained were 65.71 and 60.58lcalmol-1 respectively. This is because this specific minimisation method does not account for the strain energy within the six membered ring, whereas in the MM2 method, the individual components of the total energy are calculated.
Modelling Using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
 |
Dichlorocarbene (12) was modelled using intially the MM2 method and then PM6 using ChemBio3D MOPAC interface. Four molecular orbitals (HOMO-1, HOMO, LUMO, LUMO+1 and LUMO+2) were computed and the presense of electron density on the carbon-carbon double bonds were analysed in detail. This experiment serves to illustrate the transition from a purely classical mechanical treatment of molecule 12 to a quantum mechanical treatment which includes the wave-description of the electrons.
Orbital Control of Reactivity
The four orbitals of the Dichlorocarbene are shown below for the HOMO, HOMO -1, LUMO and LUMO +1. It can be seen that The HOMO of the alkene endo to the chlorine atom is the most nucleophilic as the PM6 calulation of this orbital has a larger coefficient at this alkene compared to the one anti to the Chloro group. Attack is found to take place at the face opposite the bridge due to steric reasons. This is explained by Halton et all [3]. An interaction between the pi bonding orbital and the carbon of the C-Cl sigma antibonding orbital produces a sigma conjugation effect. These two orbitals are antiperiplanar to each other and hence the overlap between the orbitals is quite strong. The C-Cl sigma antibonding orbital is represented by the LUMO+1 and the pi bonding orbital of the exo alkene by HOMO-1. As one can see, the symmetry of the orbitals are in good agreement for effective orbital overlap. This stabilising interaction removes electron density from the pi orbital to the sigma orbital of the C-Cl bond. This lowers the energy of the orbitals relative to the endo. Hence, the HOMO-1 orbital is seen to be more delocalised.
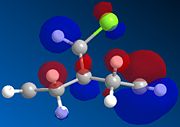 |
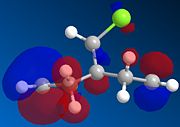 |
 |
 |
Bonding Vibrational Frequencies of the C=C and C-Cl
Two molecules of compound 12 were compared to see what influence of the Cl-C bond had on the vibrational frequencies and a Density Functional Approach (MOPAC/RM1) was used to to calculate these frequencies.
Comparison of the vibrational frequencies of the monoalkene of compound 12 with the dialkene (specifically the C-Cl and C=C stretching modes) ware shown. In both geometries, the vibrational frequencies were calculated using B3LYP/6-31G(d,p) optimisation. The 2 C=C stretching frequencies in 12 are seen at 1737 and 1757 wavenumbers for the exo and endo alkenes, respectively. These two stretching frequencies are clearly different although similar and is due to donation of electron density from the pi to sigma antibonding orbitals. This inturn weakens the double bond in the exo adduct relative to the endo and as a result, apears at a lower wavenumber.
C-Cl stretching frequenciess are also observed at 771 and 775 cm-1 for both products respectively.
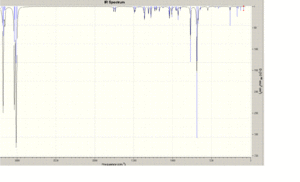 |
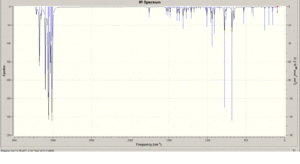 |
Structure Based Mini project using DFT-based Molecular orbital Methods
Stable phosphatoxy nitroalkanes, readily assembled by the Henry reaction and subsequent phosphorylation, serve as good precursorso to akene radical cations on treatment with triphenyltin or trityl hydride and AIBN in benzene at reflux. When the phosphatoxy nitroalkane is suitably functionalised with nucleophilic groups, substitutions can be achieved with the formation of heterocyclic rings. When the nucleophile is an allylamine, tandem processes occure giving pyrrolizidines. The reaction scheme below, shows the substrate (28) being treated with tributyltin hydride and AIBN to form a 1:1 mixture of cyclised diastereoisomers (29 and 30).
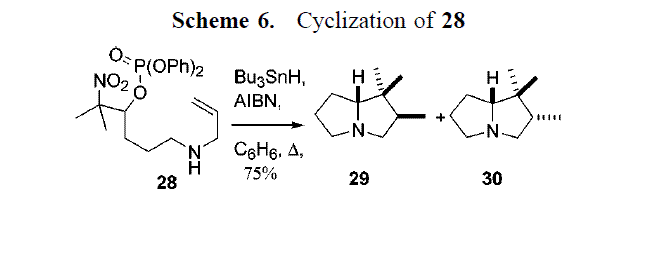
The geometries of both stereoisomers were fully optimised at the mpw1pw91/6-31(d,p) level and the GIAO method was used to predict the 13C NMR and 1H NMR of both diastereoisomers and were compared with the reference Cite error: Invalid <ref> tag; refs with no name must have content. Both isomers only differ by the stereochemistry of a methyl group, with one isomer having the methyl group going out of the plane and the other pointing into the plane or the ring.
It can be seen that the chemical shifts from the 13 C NMR of both diastereoisomers corrolate well with the reference values. However, there were a few shifts calculated with the GIAO method that were not reported in the literature.

| Diastereoisomer 29 | ||||||||||
| Calc | 17.61 | 22.76 | 23.82 | 27.31 | 29.52 | 39.47 | 48.69 | 49.07 | 56.91 | 77.20 |
| Lit | 17.8 | - | 23.3 | 26.7 | 30.1 | 41.9 | - | - | 55.6 | 75.8 |
| C assignment | 9 | 10 | 6 | 11 | 7 | 1 | 8 | 5 | 4 | 2 |
| Diastereoisomer 30 | ||||||||||
| Calc | 16.09 | 18.62 | 24.29 | 25.76 | 29.28 | 39.99 | 47.55 | 48.97 | 52.60 | 76.42 |
| Lit | 11.5 | - | 23.3 | 27.1 | 28.9 | 40.1 | - | 57.8 | 62.4 | 76.7 |
| C assignment | 2 | 4 | 5 | 8 | 1 | 7 | 11 | 6 | 10 | 9 |
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ W.F Maier and R. Schleyer, J.Am.Chem.Soc.,103,1981
- ↑ B. Halton and S.G.G Russell, J. Org. Chemistry, 1991, 56, 5553. DOI:10.1021/jo00019a015
