Rep:Mod:gm1616
EX3 section
BH3 molecule
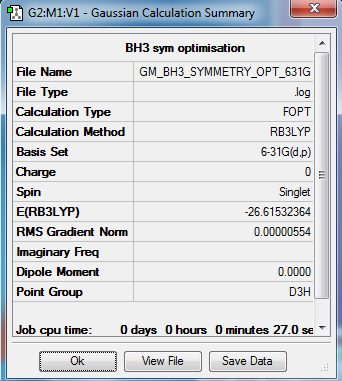
Initial BH3 analysis
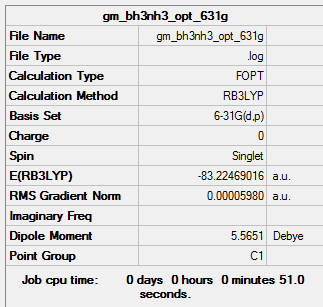
Using the B3LYP method and 6-31G(d,p) basis set:
Item Value Threshold Converged? Maximum Force 0.000011 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000044 0.001800 YES RMS Displacement 0.000029 0.001200 YES
Frequency Analysis Log File: media:GM_BH3_FREQUENCY.LOG
Low frequencies from the frequency optimized BH3:
Low frequencies --- -7.9073 -1.6385 -0.0055 0.6256 6.5697 6.7709 Low frequencies --- 1162.9662 1213.1623 1213.1650
Optimised BH3 Molecule |
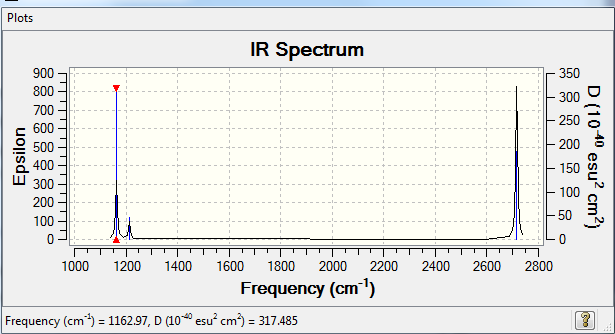
Vibrational spectrum for BH3
| Wavenumber(cm-1) | intensity | Symmetry | IR Active? | type |
|---|---|---|---|---|
| 1162.97 | 92.55 | - | Yes | Bend out of the plane |
| 1213.16 | 14.05 | - | Slightly | Bend |
| 1213.16 | 14.05 | - | Slightly | Bend |
| 2582.38 | 0.00 | - | No | Symmetric Stretch |
| 2715.56 | 126.32 | - | Yes | Asymmetric Stretch |
| 2715.56 | 126.32 | - | Yes | Asymmetric Stretch |
These allowed vibrations give a predicted spectra as shown below:
There are only 3 peaks in the IR spectrum rather than the 6 types possible because some of the vibrations are not allowed as they do not have a change in dipole which disobeys the IR selection rule. Also, some of the vibrations are degenerate at the same frequency and so they overlap and are seen as one peak rather than two.
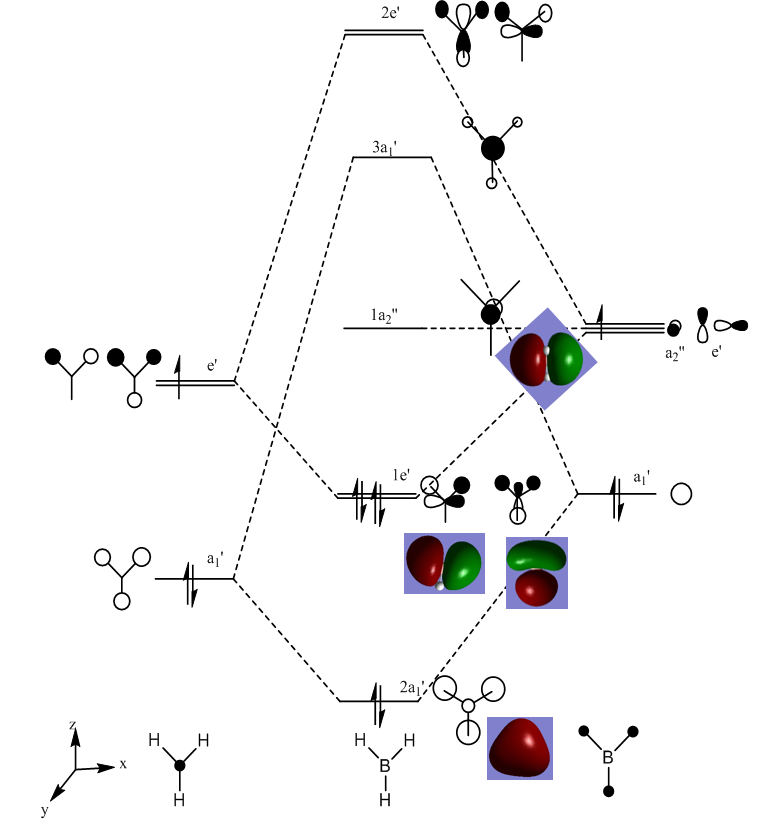
MOs of BH3
The real MO shapes obtained from the frequency calculations of BH3 are shown below alongside the LCAOs predicted from qualitative MO theory:
The differences between the real computed MOs and the LCAO MOs are minimal since the overall shapes are the same and the predicted overlap between the positive and negative lobes are similar.The main difference is the relative sizes of the combining AOs from each fragment due to different energy contributions from the fragments to the MOs. This suggests MO theory is a good model for finding the shapes and energies of real MOs.
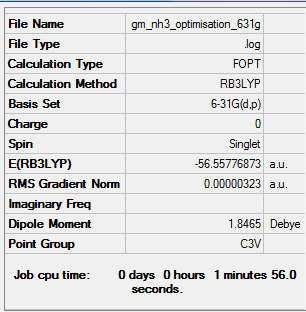
NH3 analysis
Using the B3LYP method and 6-31G(d,p) basis set:
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Frequency Analysis Log File: media:GM_NH3_FREQUENCY.LOG
Low frequencies from the frequency optimized NH3:
Low frequencies --- -8.5646 -8.5588 -0.0047 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Optimised NH3 Molecule |
NH3BH3 analysis
Using the B3LYP method and 6-31G(d,p) basis set:
Item Value Threshold Converged? Maximum Force 0.000119 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000569 0.001800 YES RMS Displacement 0.000302 0.001200 YES
Frequency Analysis Log File: Media:GM_BH3NH3_FREQ.LOG
Low frequencies --- -0.0013 -0.0007 0.0008 19.2562 23.4440 39.9371 Low frequencies --- 266.6701 632.2705 639.4988
Optimised NH3BH3 Molecule |
E(NH3)=-56.55776873 a.u.
E(BH3)=-26.61532360 a.u.
E(NH3BH3)=-83.22469016 a.u.
To find the dissociation energy:
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
ΔE=-83.22469016-[(-56.55776873) + (-26.61532360)]
ΔE=-0.05160 a.u. (5d.p.)
ΔE=-135 kJ/mol (3s.f.)
This shows that the B-N dative bond is relatively weak compared to most covalent bonds between group 1 elements which have bond energies around 200 to 400 kJ/mol.
BBr3 Analysis
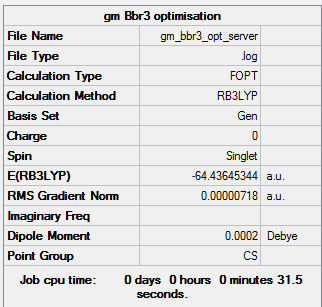
Using the B3LYP method and -Gen basis set:
Item Value Threshold Converged? Maximum Force 0.000016 0.000450 YES RMS Force 0.000008 0.000300 YES Maximum Displacement 0.000076 0.001800 YES RMS Displacement 0.000036 0.001200 YES
Frequency Dspace Link: DOI:10042/202322
Low frequencies --- -0.0001 0.0001 0.0001 1.9202 3.4554 4.5686 Low frequencies --- 155.9396 156.0232 267.7065
Optimised BBr3 Molecule |
Project Section - Main Group Halides
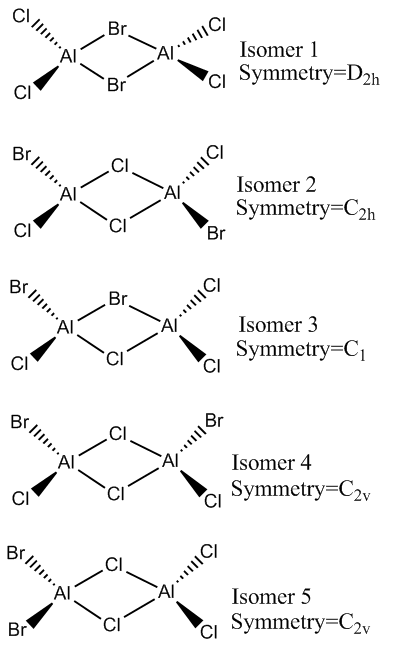
The possible Al2Cl4Br2 Isomers
Isomer 1 Analysis
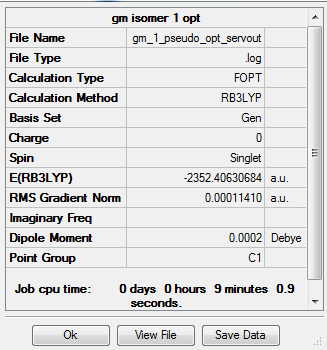
Using the B3LYP method and -Gen basis set with pseudo potentials for Br
Item Value Threshold Converged? Maximum Force 0.000149 0.000450 YES RMS Force 0.000075 0.000300 YES Maximum Displacement 0.000829 0.001800 YES RMS Displacement 0.000468 0.001200 YES
Frequency analysis log file: Media:Gm_1_pseudo_freq_servout.log
Low frequencies --- -6.0239 -5.5319 -3.2915 -0.0035 -0.0028 -0.0025 Low frequencies --- 14.2812 63.1933 86.1737
Optimised isomer 1 of Al2Cl4Br2 |
The energy for this conformer with both the Br atoms as bridging atoms is:
E=-2352.40631 a.u. (5d.p.)
E=-6175067 kj/mol (nearest integer)
Smf115 (talk) 08:49, 17 May 2018 (BST)Good structure information but detail of the pseudopotential is lacking, it isn't GEN but the combination of LanL2DZ for Br and 6-31g(d,p) for Al and Cl.
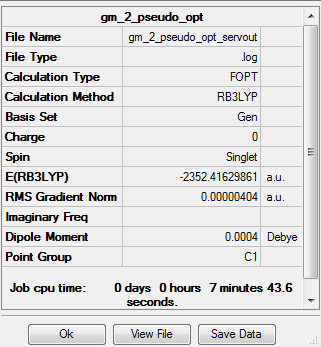
Isomer 2 Analysis
Using the B3LYP method and -Gen basis set with pseudo potentials for Br
Item Value Threshold Converged? Maximum Force 0.000005 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000054 0.001800 YES RMS Displacement 0.000024 0.001200 YES
Frequency analysis log file: media: Gm_2_pseudo_freq_servout.log
Low frequencies --- -5.1490 0.0027 0.0027 0.0036 1.4146 2.0504 Low frequencies --- 18.1472 49.1065 73.0086
Optimised isomer 2 of Al2Cl4Br2 |
The energy of this conformer with the Cl atoms as the bridging atoms and the Br atoms trans is:
E=-2352.41630 a.u. (5d.p)
E=-6175093 kJ/mol (nearest integer)
Isomer 1 vs Isomer 2
The energy difference between Isomer 1 and Isomer 2 is:
E(Isomer 1) - E(Isomer 2)
=(-6175067) - (-6175093)
= +26 kJ/mol
This shows that Isomer 2 is more stable than Isomer 1 by 26 kJ/mol since Isomer 2 has a more negative energy. The Al center has 6 electrons so it is electron deficient and uses 3c-2e bonds to relieve this deficiency and complete the octet by a dimer. This is the expected result since the chlorine atoms will be better at relieving the Al's electron deficiency than bromine since both chlorine and aluminium are in Group 3 of the Periodic table. This means they will each use there 3p orbitals to which results in a stronger orbital overlap since they are of similar sizes and energies. The 4p bonding orbital on bromine is more diffuse and so forms a weaker bond with the Al centers.
Smf115 (talk) 08:50, 17 May 2018 (BST)Correct calculation with consideration given to the accuracy of the final value. Nice explaination to justify the result.
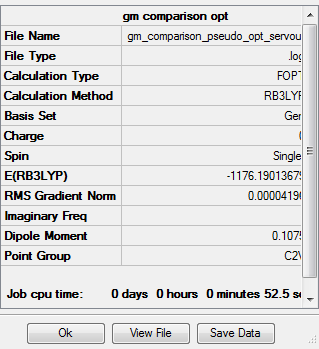
Dissociation Energy of Isomer 2 using AlCl2Br
Using the B3LYP method and -Gen basis set with pseudo potentials for Br in AlCl2Br
Item Value Threshold Converged? Maximum Force 0.000136 0.000450 YES RMS Force 0.000073 0.000300 YES Maximum Displacement 0.000760 0.001800 YES RMS Displacement 0.000497 0.001200 YES
Frequency analysis log file: Media:Gm_comparison_pseudo_freq_servout.log
Low frequencies --- 0.0028 0.0031 0.0048 1.3569 3.6367 4.2604 Low frequencies --- 120.5042 133.9178 185.8950
Optimised AlCl2Br |
The dissociation energy of Isomer 2 is:
2E(AlCl2Br)-E(Al2Cl4Br2)
= 2(-1176.19014)-(-2352.41630)
= 0.03602 a.u. (5d.p.)
= 95 kJ/mol (nearest integer)
The positive bond dissociation energy means the dimer is energetically favourable compared to the reactant monomers that from the dimer.
MOs of Isomer 2
The following MOs of Isomer 2 were analysed. None of these isomers has through bond interactions since the aluminium was the only atom that had the ability to overlap with the orbitals along the bond, yet there were no aluminium orbitals in the combined valance occupied MOs between MO25 and MO54. A through bond interaction is stronger than a through space interaction, and it often leads to directed overlap which is a stronger overlap than non-directed overlap since the p orbitals overlap 'head-on'.
Non-occupied MOs were not analysed since the electron density is polarized out of the inter nuclear region.
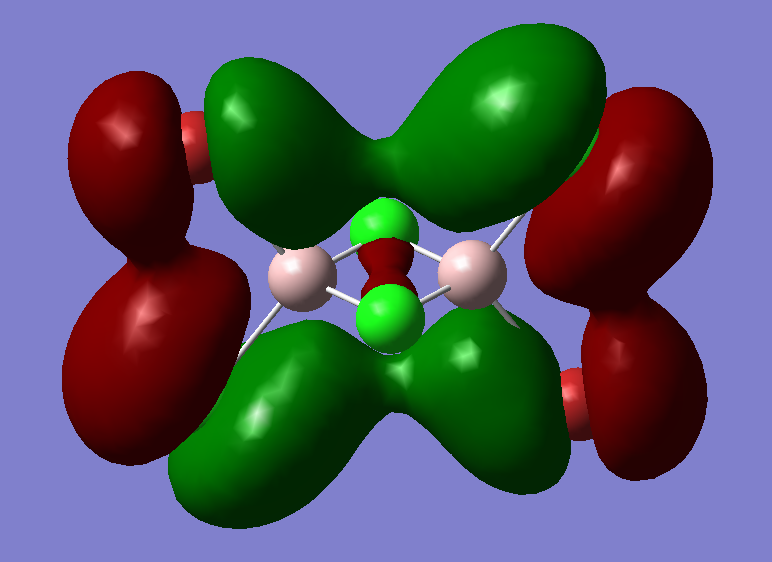
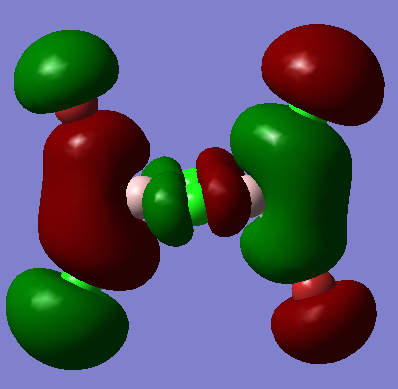
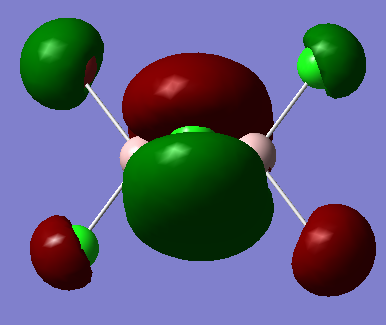
MO 47
The highly bonding MO from qualitative LCAO theory is:
The real MO is:
MO 44
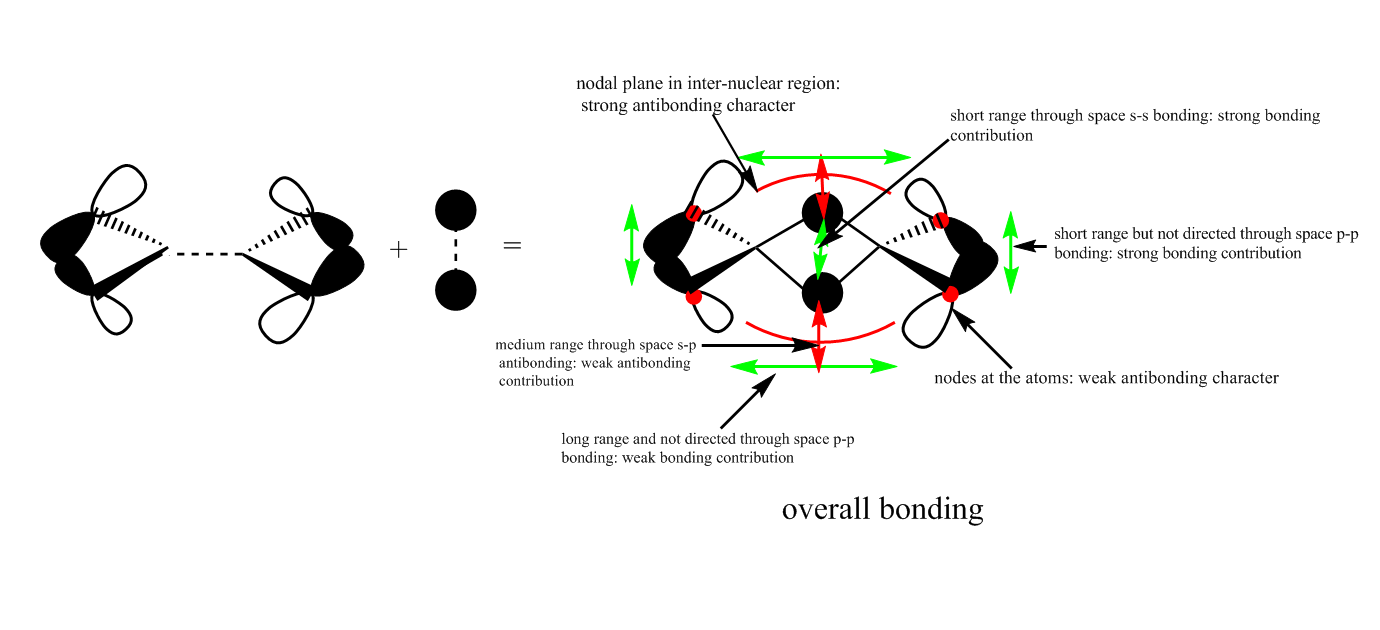
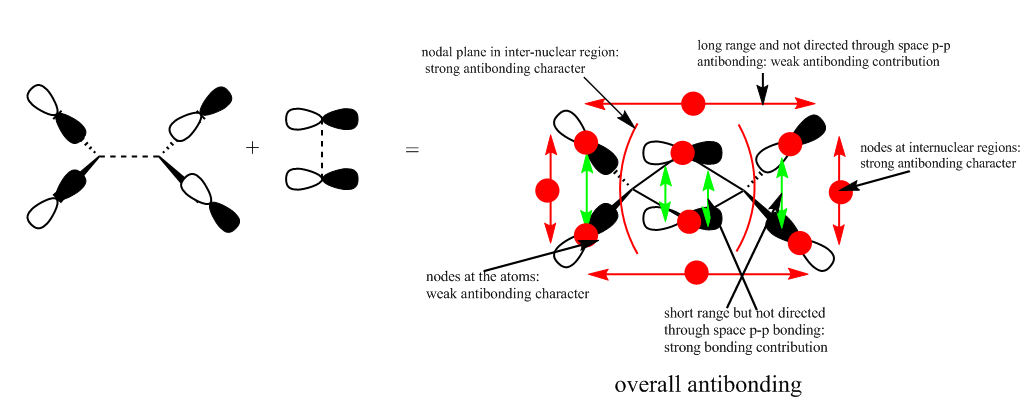
The highly antibonding MO from qualitative LCAO theory is:
The real MO is:
MO 39
Another antibonding MO from qualitative LCAO theory is:
The real MO is:
This antibonding MO has less antibonding character than MO 44 since it has less nodal planes and nodes which means it has a lower energy and has more bonding character. Also, the main antibonding is between s and p orbitals from the Br and Cl atoms which is a weaker interaction than like p-p orbital interactions from MO 44.
Smf115 (talk) 08:48, 17 May 2018 (BST)Good analysis of the MOs with the nodal planes and interactions clearly annotated. Nice inclusion of the FOs and a good range of MOs were chosen. To improve a few more details, such as size contribution or the electron desnity being polarised off the terminal atoms in MO 39 could be noticed. Overall, a clear analysis though.
Smf115 (talk) 08:51, 17 May 2018 (BST)Overall a clear and well presented wiki report with some thoughtful explainations.