Rep:Mod:giggidy
The Transition State
The Cope Rearrangement
File:Reaction mechanism.gif The mechanism of the Cope rearrangement, the [3,3]-sigmatropic rearrangement of 1,5-hexadiene, is believed to involve a chairlike transition state of C2h symmetry[1]. The mechanisms of the Cope and Claisen reactions remain a source of controversy in spite of having being probed repeatedly by experimental[2] and theoretical[3] inquiry.
Optimizing the Reactants and Products
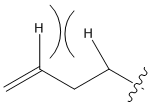
A molecule of 1,5-hexadiene was created in GaussView 5.0. The geometry was adjusted so that the central four carbon atoms were in an anti-linkage. The geometry was then optimized at the HF/3-21G level. This optimization initially returned the conformer labelled anti-3 in the table below. The geometries were then, once again, adjusted manually - this time to attain all four anti-conformers listed in Appendix 1.
| Conformer | Point Group | Energy/ hartree | Relative Energy*/ kcalmol-1 |
|---|---|---|---|
| C2 | -231.69260 | 0.04 | |
| Ci | -231.69254 | 0.08 | |
| C2h | -231.68907 | 2.25 | |
| C1 | -231.69097 | 1.06 |
*Relative Energies are relative to most stable conformation of 1,5-hexadiene - Gauche-3
Energies are in perfect agreement with those given in Appendix 1.
Another molecule of 1,5-hexadiene was created in GaussView 5.0. The geometry was adjusted so that the central four carbon atoms had a gauche linkage. The geometry was then optimized at the HF/3-21g. This optimization initially returned the conformer labelled gauche-2 in the table below. The geometries were then, once again, adjusted manually - this time to attain all six gauche-conformers listed in Appendix 1.
| Conformer | Point Group | Energy/ hartree | Relative Energy*/ kcalmol-1 |
|---|---|---|---|
| C2 | -231.68772 | 3.10 | |
| C2 | -231.69167 | 0.62 | |
| C1 | -231.69266 | 0.00 | |
| C2 | -231.69153 | 0.71 | |
| C1 | -231.68962 | 1.91 | |
| C1 | -231.68916 | 2.20 |
Energies are in perfect agreement with those given in Appendix 1.
Discussion of Relative Energies:
The stability of a given conformer of 1,5-hexadiene will be governed by steric effects and the gauche effect.
- Anti-3 is the least stable anti-conformer. This is despite the fact that it has zero dipole moment (c.f. 0.20, 0.00, and 0.29 Debye for anti-1, anti-2, and anti-4, respectively) and has no A-1,3 interactions (c.f. 2, 2, and 1 interaction(s) for anti-1, anti-2, and anti-4, respectively).
The higher energy of anti-3 must therefore be due to the two 1,4-interactions between a terminal hydrogen on the alkene and the two methylene hydrogens.
- Anti-4 has one of these 1,4-interactions (c.f. 0 interactions for both anti-1 and anti-2) and is therefore the second least stable anti-conformer.
- Anti-1 is only slightly more stable (0.04 kcalmol-1) than anti-2 and the reason for this phenomenon is less obvious. Thus, the following table has been created to try to quantify the various steric interactions:
| Interaction | Anti-1/ Å | Anti-2/ Å | Anti-3/ Å | Anti-4/ Å |
|---|---|---|---|---|
| File:Steric effect in anti1.gif | 2.45, 2.45 | 2.45, 2.45 | -, - | 2.44, - |
| File:Steric effect in anti3.gif | -, - | -, - | 2.41&2.41, 2.41&2.41 | -, 2.45&2.41 |
 |
2.64, 2.64 | 2.67, 2.67 | -, - | 2.63, - |
Note I: Two values (separated by a comma) are given for each interaction as there are two terminals and, hence, two possible interactions per molecule.
Note II: Each 1,4-interactions are given as x.xx&x.xx. This is because the methylene group has two hydrogens involved in the interaction, therefore there are two distances.
- The table does not reveal any significant differences between anti-1 and anti-2 which would explain the greater stability of anti-1.
- Gauche-1 is the least stable gauche-conformer due to the large interaction arising from forcing the two terminal =CH2 groups to share the same space.
Optimization of Anti-2 at a Higher Level
Anti-2 was re-optimized at the B3LYP/6-31G* level:
| Conformer | Point Group | Energy/ hartree |
|---|---|---|
| Ci | -234.61171 |
The following table compares the geometries of anti-2 returned by HF/3-21G and B3LYP/6-31G*:
| Method | C=C bond length/ Å | C-C bond length/ Å | H-C-H terminal alkene bond angle/ o |
|---|---|---|---|
| HF/3-21G | 1.32, 1.32 | 1.51, 1.55, 1.51 | 116.3, 116.3 |
| B3LYP/6-31G* | 1.33, 1.33 | 1.50, 1.55, 1.50 | 116.5, 116.5 |
| Percentage Change/ % | 0.8, 0.8 | 0.7, 0.0, 0.7 | 0.2, 0.2 |
As shown by the above table, the re-optimization results in only very small changes in geometry.
Vibrational Analysis of Anti-2
A frequency calculation was carried out on anti-2 at the B3LYP/6-31G* level.
| Energy | Calculated/ Hartree | Experimental |
|---|---|---|
| Sum of electronic and zero-point energies | -234.46920 | cell |
| Sum of electronic and thermal energies | -234.46186 | cell |
| Sum of electronic and thermal enthalpies | -234.46091 | cell |
| Sum of electronic and thermal free energies | -234.50078 | cell |
Optimizing the Chair Transition Structure
An allyl fragment was created in GaussView and then optimized at the HF/3-21G level. This fragment was duplicated, and a guess chair structure was created. This guess transition structure was then optimized via the following two methods:
| Method | Bond-forming~bond-breaking lengths/ Å |
|---|---|
| 2.02, 2.02 | |
| 2.23, 2.24 |
The calculation yields one imaginary frequency at 818 cm-1, which corresponds to the Cope rearrangement.
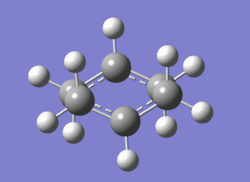
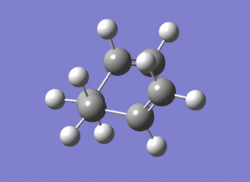
The Chair transition state (on the left) clearly corresponds to the gauche2 conformer (on the right) from earlier.
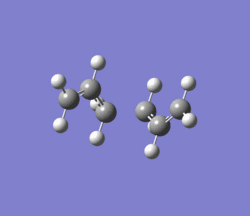
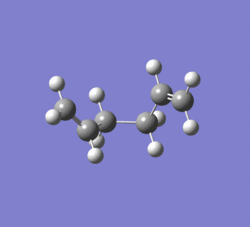
IRC (left) was used to confirm that the correct conformer had been assigned to the chair transition state. The two images nearly match eachother - if conformer image matches the mirror-image of the transition state image. This suggests that they are describing the breaking/formation of opposite bonds i.e. whilst one breaks/forms, the other forms/breaks.
Optimizing the Boat Transition Structure
The boat transition structure was optimized using the QST2 method using the Ci anti-2 conformer. The anti-2 conformer was initially optimized at the B3LYP/6-31G* level, and so the QST2 calculation was also carried out at the B3LYP/6-31G* level.
The transition structure has one imaginary frequency at 531 cm-1, which corresponds to the Cope rearrangement.
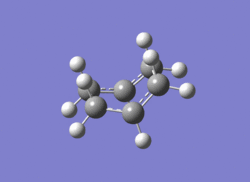
The boat transition state (left) corresponds to the gauche-1 conformer (right) from earlier.
Determination of Activation Energies
When calculating the activation energies, the most stable conformer gauche-3 will be used as a reference.
| Molecule | Energy/ a.u. | Activation Energy/ kcalmol-1 |
|---|---|---|
| gauche-3 | -234.61133 | - |
| Chair | -234.55698 | 34.10 |
| Boat | -232.87648 | 1088.63 |
