Rep:Mod:gc517
NH3 molecule
Geometric properties
- N-H Bond lenght = 1.01791 Å
- H-N-H Bond Angle = 105.741°
The nitrogen atom has 4 electron pairs; among them, 3 are involved in the bonding with the hydrogen atom. The lone pair exerts a greater repulsion than the bonding pair, resulting in an angle of 105.741° and an overall trigonal pyramidal shape.
Gaussian Calculation Summary
- Calculation Method = RB3LYP
- Basis Set = 6-31G(d,p)
- Final Energy = -56.55776873 a.u.
- RMS Gradient = 0.00000485 a.u.
- Point Group = C3v
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986275D-10
Optimization completed.
-- Stationary point found.
media:GIULIAC PHUNT NH3 OPTF POP.LOG
NH |
Vibration modes
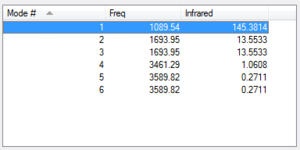
According to the 3N-6 rule for vibrational modes, 6 vibrations are expected for a molecule of ammonia. As it can be observed from the table above, four of them are degenerate (i.e. have the same energy): the N-H-N scissoring (modes 2 and 3) and the N-H asymmetric stretching (modes 5 and 6). Modes 1, 2 and 3 are bending vibrations, while modes 4, 5, and 6 are stretching vibrations. Among them, mode 4 is a symmetrical stretching, whereas the bending mode 1 is also known as the "umbrella" one.
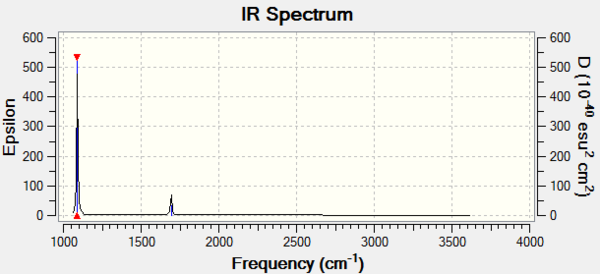
The Infrared column in the table gives the Intensity of the peaks in a IR spectrum: for the molecule of ammonia considered, only 2 peaks will be observed in the experimental spectrum of the gaseous molecule, one for mode 1 and one for the degenerate modes 2 and 3. Modes 4, 5 and 6 have a too low intensity to appear in the spectrum.
Charge Analysis
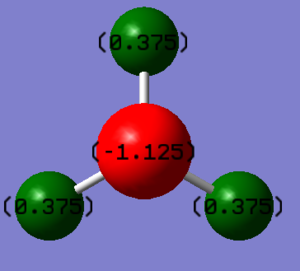
- Charge on N atom = -1.125 e
- Charge on H atom = 0.375 e
In the ammonia molecule, the N atom is more electronegative than H and, therefore, it pulls the electron density towards itself. The sum of the charges of the 3Hs counterbalance the negative charge on N, resulting in a neutral molecule.
N2 molecule
Geometric properties
- N-N Bond lenght = 1.10550 Å
Gaussian Calculation Summary
- Calculation Method = RB3LYP
- Basis Set = 6-31G(d,p)
- Final Energy = -109.52412868 a.u.
- RMS Gradient = 0.00000060 a.u.
- Point Group = D∞h
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401036D-13
Optimization completed.
-- Stationary point found.
media:GIULIAC PHUNT N2 OPTF.LOG
N |
Vibration modes
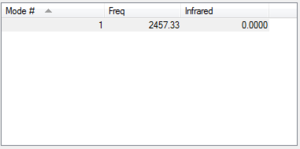
N2 is a linear molecule. Therefore only 1 vibrational mode is expected (3N-5). This is a stretching vibration. However, as it can be seen from the table, this vibration is IR inactive (there is no change in dipole).
Charge Analysis
- Charge on N atom = 0.000 e
In the N2 molecule, the 2 N atoms have the same electronegativity, therefore the charge distribution is 0.
H2 molecule
Geometric properties
- H-H Bond lenght = 0.74279 Å
Gaussian Calculation Summary
- Calculation Method = RB3LYP
- Basis Set = 6-31G(d,p)
- Final Energy = -1.17853936 a.u.
- RMS Gradient = 0.00000017 a.u.
- Point Group = D∞h
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
media:GIULIAC PHUNT H2 OPTF.LOG
H |
Vibration modes
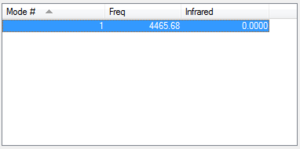
H2 is a linear molecule. Therefore only 1 vibrational mode is expected (3N-5). This is a stretching vibration. However, as it can be seen from the table, this vibration is IR inactive (there is no change in dipole).
Charge Analysis
- Charge on H atom = 0.000 e
In the H2 molecule, the 2 H atoms have the same electronegativity, therefore the charge distribution is 0.
Reaction Energy for the Haber-Bosch process
N2 + 3H2 -> 2NH3
- E(NH3) = -56.55776873 a.u.
- 2*E(NH3) = -113.1155375 a.u.
- E(N2) = -109.52412868 a.u.
- E(H2) = -1.17853936 a.u.
- 3*E(H2) = -3.53561808 a.u.
- ΔE=2*E(NH3)-[E(N2)+3*E(H2)] = -0.0557907 a.u. = -146.48 kJ/mol
The Haber-Bosch process is thermodinamically favourable: the reaction is, in fact, exothermic (ΔE<0). The ammonia produced is lower in energy, hence more stable, than the gaseous reactants used.
CH4 molecule
Geometric properties
- C-H Bond lenght = 1.09197 Å
- H-C-H Bond Angle = 109.471°
The H-C-H angle is reflected in the tetrahedral shape of the methane molecule.
Gaussian Calculation Summary
- Calculation Method = RB3LYP
- Basis Set = 6-31G(d,p)
- Final Energy = -40.52401404 a.u.
- RMS Gradient = 0.00003263 a.u.
- Point Group = Td
Item Value Threshold Converged?
Maximum Force 0.000063 0.000450 YES
RMS Force 0.000034 0.000300 YES
Maximum Displacement 0.000179 0.001800 YES
RMS Displacement 0.000095 0.001200 YES
Predicted change in Energy=-2.256038D-08
Optimization completed.
-- Stationary point found.
media:GIULIAC PHUNT CH4 OPTF.LOG
CH4 |
Vibration modes
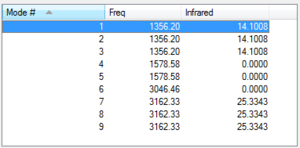
According to the 3N-6 rule for vibrational modes (methane is a non-linear molecule), 9 vibrations are expected for a molecule of methane. As it can be observed from the table above, six of them are degenerate (i.e. have the same energy): the N-H-N scissoring (modes 1,2 and 3) and the C-H asymmetric stretching (modes 7,8 and 9). Modes 1 to 5 are bending vibrations, while modes 6 to 9 are stretching vibrations.
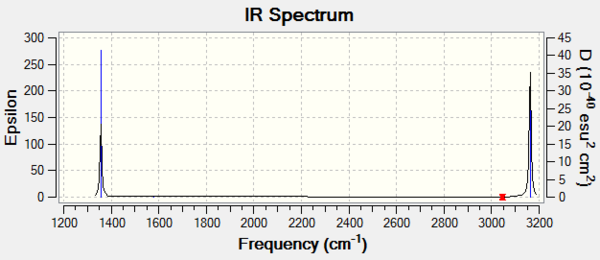
The IR spectrum of methane has only 2 peaks, one for modes 1,2 and 3 and one for modes 7,8 and 9. Modes 4, 5 and 6 will only be visible in the Raman spectrum.
Charge Analysis
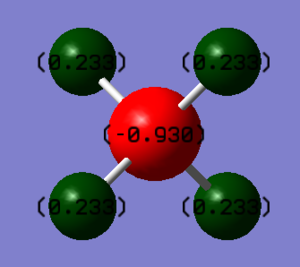
- Charge on C atom = -0.930 e
- Charge on H atom = 0.233 e
Molecular Orbitals Analysis
The electronic configuration of a carbon atom is 1s22s22p2. Theoretically, carbon would hence form 2 bonds through the 2 unpaired electrons in the 2p orbital. However, it is experimentally observed that carbon is tetravalent. This phenomenon can be rationalised with hybridisation.
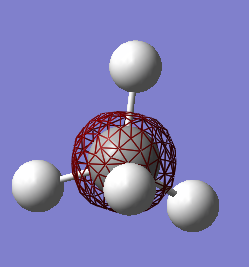
As it can be observed in the table of the MO's energies, the 1s orbital of carbon is too low in energy to interact with the 1s orbital of hydrogen. It is, therefore, held tightly to the carbon nucleus.
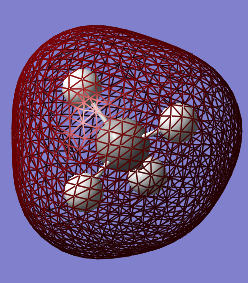
MO 2 corresponds to the combination of the hybridised 2s orbital of carbon and the 1s AO of hydrogen. Its energy is much higher than the one of MO 1 and the extended surface is the result of the strong overlap between the two AOs of carbon and hydrogen.
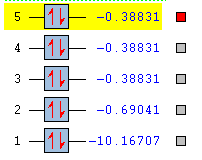
The next 3 MOs are the sp3 orbitals, a combination of 25% s and 75% p character that mix with the 1s orbitals of 3 atoms of hydrogen. As it can be seen from the table of energies, these orbitals possess all the same energies and are said to be degenerate
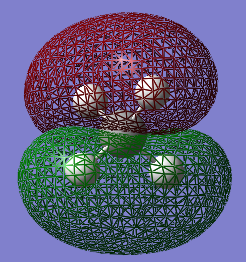
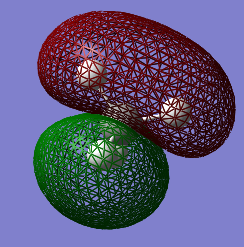
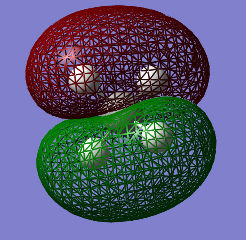
The 10 electrons in the methane molecule (6 coming from C and 4 from the 4 atoms of H) are accommodated in the first 5 MOs. MO 5 corresponds to the HOMO.
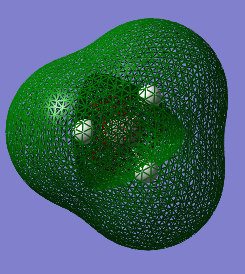
MO 6 is the LUMO and corresponds to the antibonding orbital of MO 2.
