Rep:Mod:fzm18
NH3, also known as ammonia, is a major compound of our curernt society, from its use in agriculture, in refrigeration... In the following sections, the main characteristics of ammonia, H2, N2 are investigated in order to study the Haber-Bosch process and why it is energetically favoured.
Finally, a comparison of cyanide [CN]- and hydrogen cyanide HCN is carried out, in order to find the most table structure between the base and the acid.
NH3 molecule
Optimization
Molecule name : NH3
Calculation method : RB3LYP
Basis set : 6-31G(d.p)
Final energy : E(RB3LYP) = -56.55776873 a.u.
RMS gradient = 0.00000485 a.u.
Point group : C3v
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
NH3 molecule |
Recorded optimized N-H bond length : rNH = 1.018 Å
Recorded optimized H-N-H bond angle : θ = 105°
The optimization file can be accessed here.
Vibration and Charges
Vibration
| Wavenumber cm-1 | 1089 | 1693 | 1693 | 3461 | 3589 | 3589 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity arbitrary units | 145 | 14 | 14 | 1 | 0 | 0 |
| Image |  |
 |
 |
 |
 |

|
From the 3N-6 rule, 6 vibrational modes are to be expected. On the table, it is shown that 4 modes are degenerate (2 of them have a frequency of 1693 cm-1, and two others have a frequency of 3589 cm-1).
The first three vibrational modes (in the table) correspond to bending, while the last three correspond to streching vibrations. The vibrational mode with a frequency 3461 cm-1, also called the symmetric strech, is the most symmetric vibration. The vibration with the smallest frequency is known as the umbrella mode.
From the degeneracy of the modes, 4 peaks should be expected in an experimental spectrum of gaseous ammonia. However, the last two signals have very low intensity and for this reason, only 2 peaks can be observed in an IR spectrum of NH3 (1089 and 1693 cm-1).
Charge
As expected, the calculations show a negative charge on the N atom, while positive chrges can be observed on the H atoms. Futhermore, it can be noted that qN = -3qH as the overall charge of the molecule Q = qN +3qH = 0.

N2 molecule
Molecule optimization
Molecule name : N2
Calculation method : RB3LYP
Basis set : 6-31G(d.p)
Final energy : E(RB3LYP) = -109.52412868 a.u.
RMS gradient = 0.00000060 a.u.
Point group : D∞h
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
N2 molecule |
Recorded N-N bond length : rN2 = 1.106 Å
Please note that N2 is a linear diatomic molecule, therefore no optimzed angle is recorded.
The optimization file can be accessed here.
Vibrations and Charges
Vibrations
Here the table shows the only vibration in N2 has a frequency of 2457 cm-1, not shown in the IR spectrum (as its intensity is predicted to be 0). This can be explained by the fact that symmetrical vibrations are not IR active.

Charge
As, N2 is a symmetric diatomic molecule, it comes to no surprise that no charge can be observed on either of the atoms, as it is displayed in the modelisation below.
H2 molecule
Molecule optimization
Molecule name : H2
Calculation method : RB3LYP
Basis set : 6-31G(d.p)
Final energy : E(RB3LYP) = -1.17853936 a.u.
RMS gradient = 0.00000908 a.u.
Point group : D∞h
Item Value Threshold Converged? Maximum Force 0.000016 0.000450 YES RMS Force 0.000016 0.000300 YES Maximum Displacement 0.000021 0.001800 YES RMS Displacement 0.000029 0.001200 YES
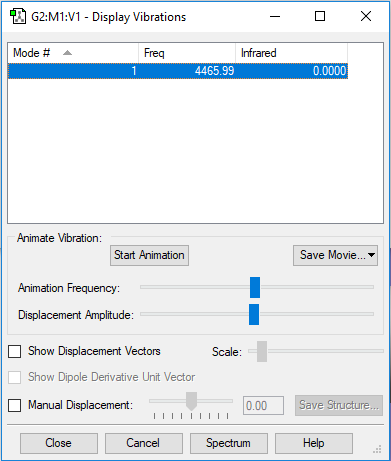
H2 molecule |
Recorded N-N bond length : rH2 = 0.743 Å
Please note that H2 is a linear diatomic molecule, therefore no optimzed angle is recorded.
The optimization file can be accessed here.
Vibrations and Charges
Vibrations
Here the table shows the only vibration in H2 has a frequency of 4466 cm-1, not shown in the IR spectrum ( as its intensity is predicted to be 0). This can be explained by the fact that symmetrical vibrations are not IR active.

Charge
As, H2 is a symmetric diatomic molecule, it comes to no surprise that no charge can be observed, as it is the case for N2.
Reaction and orbitals
Structure and reactivity
Mono-metallic transition metal complex
A search in ConQuest permits to find that Molybdenum compound coordinates N2: cis-bis(1-(diethylphosphino)-N-((diethylphosphino)methyl)-N-(2,6-difluorobenzyl)methanamine)-bis(dinitrogen)-molybdenum. Its unique identifier is AQEZED, and more information about the compound can be found |here.
The recorded bond length in that molecule are r1 = 1.117 Å and r2 = 1.113 Å. Those values are higher than the predicted bond length calculated on Gaussian. This can be explained by the fact that the first N atom also shares a bond with the Mo atom, leading to a more diffuse distribution of the electrons around that nitrogen atom (formal positive charge), hence the difference between the computed and experimental bonds.
Haber-Bosch process
E(NH3) = -56.55776873 a.u.
2*E(NH3)= -113.1155375 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579074 a.u. = -146.5 kJ.mol-1 (tp 1 d.p.)
ΔE is negative, indicating that the product (ammonia) is more stable than the reactants (nitrogen and hydrogen).
Cyanide
In this part, we will be intersted in the [CN]- molecule, a very harmful substance to living beings, yet impressively useful molecule at a catalysist or base in different reactions.
[CN]- molecule
Molecule optimization
Molecule name : [CN]-
Calculation method : RB3LYP
Basis set : 6-31G(d.p)
Charge : q = -1
Final energy : E(RB3LYP) = -92.82453153 a.u.
RMS gradient = 0.00000704 a.u.
Point group : C∞v
Item Value Threshold Converged?
Maximum Force 0.000012 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.000005 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
[CN]- molecule |
Recorded bond length : rCN = 1.184 Å
[CN]- being a linear diatomic molecule, no optimized angle was calculated.
The optimization file can be accessed here.
Vibrations and charges
Vibrations
[CN]- being a diatomic molecule, it only shows one vibration mode : the C-N stretch, and thus its IR spectrum will be composed of a unique peak at 2139 cm-1, and of intensity 7 (in arbitrary units). This vibration is illustrated by the figure below.
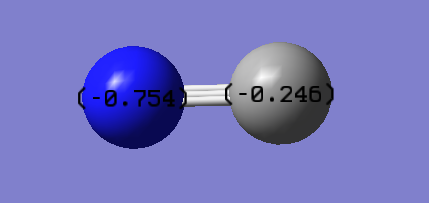
Charge
[CN]- is an anion with a charge q = -1, which is, in theory, on the C atom. However, according to computed calculations, the strong electronegative nature of the nitrogen atom results on a distribtion of the negative charge throughout the molecule, with a great portion of it still on the C atom. This is can be illustrated by the picture below:
HCN molecule
The negative charge on [CN]-</sup< can be neutralized when the carbon atom bonds with a Hydrogen atom, forming a HCN molecule. Let us study this new molecule.
Molecule optimization
Molecule name : HCN
Calculation method : RB3LYP
Basis set : 6-31G(d.p)
Final energy : E(RB3LYP) = -93.42458132 a.u.
RMS gradient = 0.00017006 a.u.
Point group : C∞v
Item Value Threshold Converged?
Maximum Force 0.000370 0.000450 YES
RMS Force 0.000255 0.000300 YES
Maximum Displacement 0.000676 0.001800 YES
RMS Displacement 0.000427 0.001200 YES
HCN molecule |
Recorded C-H bond length : rC-H = 1.069 Å
Recorded C-N bond length : rC-N = 1.157 Å
Recorded bond angle : θ = 180°
The optimization file can be accessed here.
Vibrations and Charges
Vibrations
| Wavenumber cm-1 | 766 | 766 | 2214 | 3479 |
| Symmetry | PI | PI | SG | SG |
| Intensity arbitrary units | 35 | 35 | 2 | 57 |
| Image |  |
 |
 |

|
Here the table shows the different vibration modes of HCN. It can be observed that the first two vibration modes are degenerate, i.e. have the same energy. The high symmetrical nature of the third onehas a very low intensity, implying that the spectrum will only show two peaks.
Charge
The Nitrogen atom is known to be highly electronegative compared to C and H, which explains its partial negative charge, while the Carbon and Hydrogen atom positive charges sum up to compensate this negative charge.
Molecular orbitals
Above is displayed a table showing five of the molecular orbitals that constitute HCN.
The first orbital chosen, bond between the H and C atoms, helps illustrating the mixing between and s and a p orbital in the C atom. The second one, 3σg, is interesting because it is part of the C-N triple bond. Its corresponding antibonding orbital should be studied as it shows, again, the mixing of the carbon atomic orbitals. Here, on the π molecular orbitals were displayed to allow to understand the degeneracy of those orbitals which also consitute the triple bond, and allows us to comprehend that the bond strength is mainly due to the efficient overlap between the 2p orbitals of the same size (as they belong to the same period). It is also the HOMO. Finally, its corresponding aintibonding orbital is shown here for similar reasons, and also because it is the LUMO.
Analytical comparison
The point of this section is to analyse the effect of the addition of a proton on the cyanide molecule.
The first point of comparison to take into consideration is the bond length of the C-N triple bond. It should be noted that the diffrence between the two bonds is :
Δr = rCN - rHCN = 1.184 - 1.157 = 0.024 Å
The bond is shorter in HCN, and thus it can be concluded that the bond is strengthened by the addition of hydrogen. This can be explained bt the fact that in [CN]-, the lone pair of electrons it very repulsive – more repulsive than a potential C-H bond, and therefore weakens the C-N bond.
HCN, more stable than [CN]- (due to the stabilising of the negative charge), has a pKa = 9.1 in water [1]. As the carbon loses its lone pair, the molecule cannot react as a base anymore, but behaves as an acid in aqueous conditions (with a pH < 9.1), as it is the case in the reaction shown below:
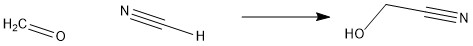
In this reaction, HCN reacts with H2C=O to form hydroxyacetonitrile. The reagent used is H2SO4. This example helps see clearly how the addition of hydrogen to cyanide makes it an efficient acid. [2]. However, it should be noted that HCn is rarely used because of its highly poisonous character, and substitutes such as KCN, or NaCN are often used.
References
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES - only in the first sentence there are some poorly formatted words.
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - you could have included a electronegativity argument to explain the charges.
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES
The third displayed MO is a bonding orbital rather than anti-bonding. Besides that you correctly described the MOs. You could have explained and discussed the relative orbital energies.
Independence 0/1
If you have finished everything else and have spare time in the lab you could:
Check one of your results against the literature, or
Do an extra calculation on another small molecule, or
YES - however the link to the .log file of the CN- ion is not working, therefore the achievable mark is lowered by 1 for this section. But you had a very good and unique idea for this section of the lab!
Do some deeper analysis on your results so far