Rep:Mod:fs1309
Year 3 Computational lab report by feng shen
Part 1
1) The Hydrogenation of Cyclopentadiene Dimer
The Hydrogenation of Cyclopentadiene According to the optimizations, the endo form is the thermodynamically more stable product compared to the exo form. But during the formation of dimer, kinetics factor (the activation energy) plays a much more important role and contradicts the thermodynamic prediction. In this case, the endo transition state is stabilised by the secondary orbital interactions between ᴨ orbitals, while such stabilisation interaction does not happen in the case of exo transition state. Therefore instead of forming the more thermodynamically stable exo product, the reaction proceeds via the pathway with the lower activation energy and forms the endo product.

| Contribution of total energy kcal/mol | product 3 | product 4 | |
|---|---|---|---|
| Stretch | 1.28 | 1.10 | |
| Bend | 19.87 | 14.52 | |
| Stretch-bend | -0.83 | -0.55 | |
| Torsion | 10.81 | 12.5 | |
| Non-1,4 VDW | -1.23 | -1.07 | |
| 1,4-VDW | 5.63 | 4.51 | |
| Dipole/dipole | 0.16 | 0.14 | |
| Total | 35.69 | 31.15 |
As the table shown above suggest, the bend is the most important factor that affect the stability. The reason for the energy difference arises from the position of double bond present in the molecule. As in such 5 or 6 member ring system, the bond angle of a double can no longer maintain as 120° degree and therefore torsional strain and bend arise. The smaller the deviation between actual bond angle and 120°, the more stable the product. According to the bond angle measurement, product 4 has the bond angle of 112°, which is closer to 120° than product 3 does and therefore it’s lower in energy.
| product 3 | product 4 |
|---|---|
 |
 |
Reference: 1. Notes from Professor Henry Rzepa on http://www.ch.ic.ac.uk/local/organic/conf/ 19/10/2011
2) Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol.
| product 9 | product 10 | ||
|---|---|---|---|
| Total energy (Kcal/mol)(MM2) | 47.84 | 53.16 | |
| Total energy (Kcal/mol)(MMFF94) | 70.55 | 77.87 |
Further minimization by manually editing the structure was not successful as the same structure was obtained after MM2 optimization for all manual editions.
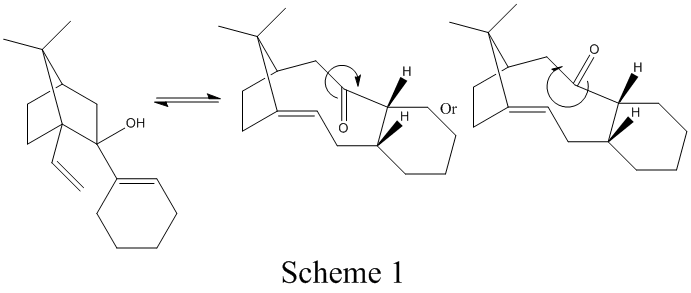
From MM2 Minimization,product 9 is more stable compared to product 10. Two different results were obtained from MM2 and MMFF94 because different factors are encounter in these 2 different methods. The reasons why alkenes react so slowly is due to the hyper stable nature of the olefins and the presence of equilibrium process as shown on the right. Also the olefin becomes hyper stable as it has less strain than the parent hydrocarbon and therefore and shows decreased reactivity because of the bridgehead location of the double bonds. Such energy difference is termed as olefin strain energy and can be used to interpret and predict the stability and reactivity of the bridgehead alkene.
- S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319
- See J. G. Vinter and H. M. R. Hoffman, J. Am. Chem. Soc., 1974, 96, 5466
- Another well known example is within Vancomycin: J. Am. Chem. Soc., 1999, 121, 3226
- An interesting variation is of "atropenantioselective cycloetherification" (G. ÊIslas-Gonzalez, M. ÊBois-Choussy and J. ÊZhu, Org. Biomol. Chem., 2003, 30-32.
- First paper formally recognizing the new class of "hyperstable" olefins (Wilhelm F. Maier, Paul Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891
- David K. Johnson and Bradford P. Mundy Tetrahedron Letters Volume 30, Issue 48 , 1989, Pages 6633-6636
- Wilhelm F. Maier, Paul Von Rague Schleyer J. Am. Chem. Soc., 1981, 103 (8), pp 1891–1900
3) Regioselective Addition of Dichlorocarbene
Section 1
| orbital | 3D model from gaussian | |
|---|---|---|
| HOMO | 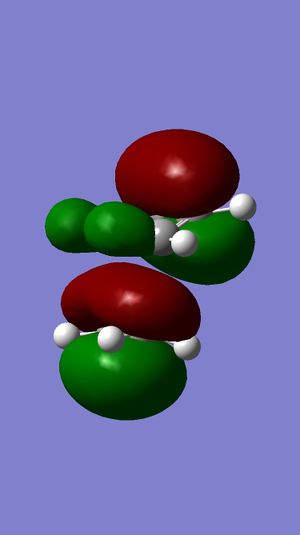 |
|
| HOMO-1 | 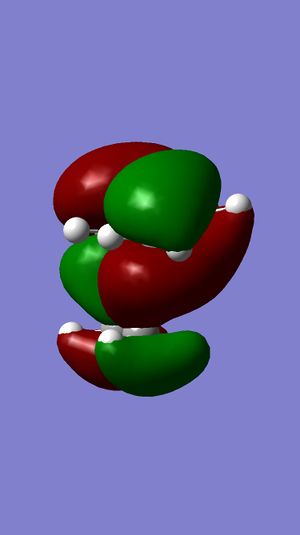 |
|
| LUOMO | 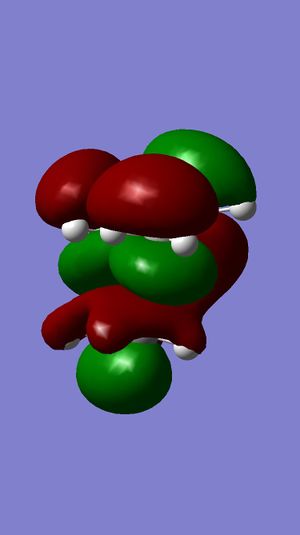 |
|
| LUMO+1 | 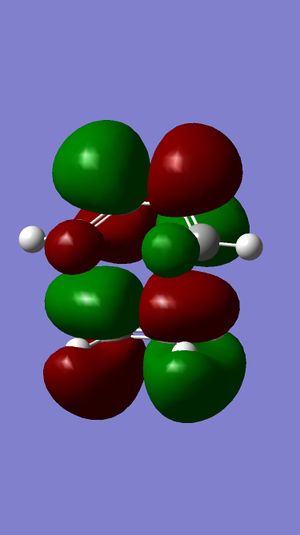 |
|
| LUMO+2 | [[Image:LUMO+2.jpg|thumb|widthpx|] |
Surface of HOMO obtained from MOPAC method suggests that the double bond endo to the chloride substituent is the most nucleophilic. This is due to the much more diffuse pi* orbital endo to chloride, which results in a better interactions between eletrophile and the double bond, compared to the other double bond.
Section 2 The influence of C-Cl bond on the vibrational frequencies of the molecule.
IR prediction from Gaussian.
Mono Alkene

Diene

| Functional group | Diene | alkene | |
|---|---|---|---|
| C-Cl | 693 | 672 | |
| C=C | 1736 and 1757 | 1757 |
The IR spectra of diene and alkene are different due to the interaction between Cl substituent and the double bond endo to it. Which results in differences in the C-Cl bond and C=C (endo to Cl) IR frequencies. The difference between two double stretching frequecnies arise from the stabilizing antiperiplanar interactions, which strengthens the exo double bond, between the C-Cl σ* orbital and the occupied anti filled pi orbital. This makes the endo double bond more reactive and therefore matches the analysis in part 1.
Reference: B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447
4)Monosaccharide chemistry: glycosidation
1: A methyl group is chosen here to keep the computational demand minimal. Hydrogen atom can not be used here as it tends to form hydrogen bonds between adjacent hydroxyl group. The MM2 method is used here as it also includes interaction between oxygen lone pairs and a better simulation of molecular modelings are obtained using MM2.
From the table above, the more stable isomers are A and B, they are both simliar to the intermediate in structure.
2: Comparison between C and D.
| Intermediate | Conformations | Energy (Kcal/mol) | Stabilization energy=Energy of A-C / B-D (Kcal/mol) | |
|---|---|---|---|---|
| C |  |
31.07 | 32.23-31.07=1.16 | |
| D |  |
52.17 | 54.12--52.17=1.95 |
![]() [[media:|...Measurements of bond angles and bond lengths using MM2 and MOPAC/PM6 method ...]]
[[media:|...Measurements of bond angles and bond lengths using MM2 and MOPAC/PM6 method ...]]![]()
As the results suggest, both methods show similar results in measuring C-H and C-C bond and they are very close to the optimal data. The difference arises in the case when oxygen get involved, the PM6 method has a greater deviation than the MM2 method does.
Due to the existence of the new 5 member ring, the only possible isomers for C and D may arise from the rotation of C-C bond between C6 and C13.
| Isomers | Conformations |
|---|---|
| C' |  |
| D' |  |
Part 2 MIni project
Regio- and stereoselective conversion of alkenes to epoxides
Both 1H NMR and IR spectroscopy techniques can be used here to identify which double bond has been epoxidised.

1) For 1H NMR, only 1 H peak will appear in the alkene hydrogen region if double bond 1 has been epoxidised, while 2 H peaks will appear within the same region if the epoxidation takes place at double bond 2.
2) For the IR spectrum. two different C=C double bond stretches can be observed for the 2 diastereoisomers. This is because the electronegative bromine on double bond 2 can withdraw the electron density from the double bond and results in a weaker bond and therefore lower frequency compared to the other double bond does.
13C NMR prediction using Gaussian:

| Number of carbon atoms in 14 | Chemical shift / ppm | Number of carbon atoms in 15 | Chemical shift / ppm | comparison to the literature /ppm | |
|---|---|---|---|---|---|
| 6 | 130.00 | 6 | 130.00 | 129.9 | |
| 1 | 131.97 | 1 | 131.97 | 126.5 | |
| 11 | 112.79 | 11 | 112.79 | 111.4 | |
| 5 | 78.04 | 5 | 78.04 | 74.2 | |
| 4 | 77.40 | 4 | 77.40 | 72.7 | |
| 3 | 52.17 | 3 | 52.17 | 49.5 | |
| 2 | 51.36 | 2 | 51.36 | 48.3 | |
| 13 | 32.25 | 13 | 32.25 | 27.5 | |
| 12 | 32.20 | 12 | 32.20 | 26.0 |
The NMR predictions on 13C NMR using Gaussian match the literature very well.
The two diastereoisomers obtained can be explained by analyzing the intermediates of the reaction. The mCPBA or NBS will first attack on one side of the alkene system. If the electrophile attacks from the same side of the acetal group, there will be steric hindrance and electrostatic repulsion between oxygen (mCPBA) or Br (NBS) lone pairs and acetal oxygen lone pairs as shown in the table below. This will result in high kinetic barriers for the reaction to proceed via this pathway. While such interactions in the case of electrophilic attack from the other side of alkene do not exist, the reaction prefers to proceed via a pathway with lower activation energy. The reaction using mCPBA stops at this point by forming epoxide, the other reaction with NBS form a bromonium, which follows by rear site nucleophilic attack by hydroxide ion, after which the oxygen can use its lone pair to replace the adjacent bromine and ends up with epoxide. (see mechanism shown on the right)
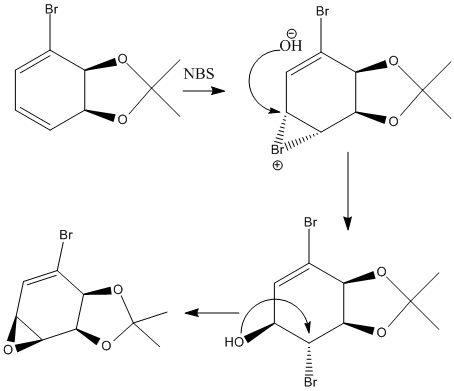
| Reagents | Model | |
|---|---|---|
| mCPBA |  |
|
| NBS |  |
Reference
- Germán Fonseca, Gustavo A. Seoane Tetrahedron: Asymmetry Volume 16, Issue 7, 4 April 2005, Pages 1393-1402
- T. HUDLICKY,F. RULIN,T. TSUNODA,H. LUNA,C. ANDERSEN,J. D. PRICE, Israel journal of chemistry,Volume 23, Issue 29, page no, July 21, 1992