Rep:Mod:frisbee0702part2
This is the second part of the organic module. The first part can be found at Mod:frisbee0702
Room Temperature Hydrolysis of a Peptide Bond
Introduction
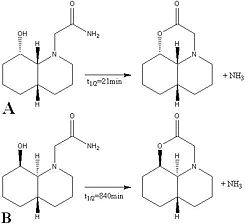
The hydrolysis of a peptide bond has a half life of approximately 500 years[1] at room temperature, however there are cases[2][3] of this occuring in a considerably shorter time. In the example given below isomer A hydrolysises 40 times faster than than isomer B. This section attempts to rationalise this differance.
Results and Analysis
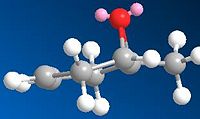
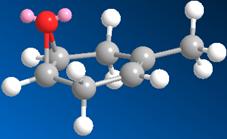
Both starting materials contain a bicyclic ring system which can be either cis (isomer A) or trans (isomer B) fused. The hydroxy group has defined stereochemistry in the starting material due to restricted bond rotation within the ring, however the peptide group does not. It is able interconvert between its axial and equatorial conformers as the ring nitrogen is able to undergo inversion. Therefore there are a total of four isomers that need to be considered.
|
Isomer A (equatorial)
Stretch: 1.7279 Bend: 5.1821 Stretch-Bend: 0.5353 Torsion: 9.3507 Non-1,4 VDW: -7.3689 1,4 VDW: 10.0094 Dipole/Dipole: -6.3127 Total Energy: 13.1237 kcal/mol |
|
Isomer A (axial)
Stretch: 1.6668 Bend: 8.3292 Stretch-Bend: 0.6453 Torsion: 11.8926 Non-1,4 VDW: -7.9460 1,4 VDW: 10.1734 Dipole/Dipole: -4.9381 Total Energy: 19.8232 kcal/mol |
|
Isomer B (equatorial)
Stretch: 1.5382 Bend: 3.2325 Stretch-Bend: 0.4867 Torsion: 7.2295 Non-1,4 VDW: -8.1081 1,4 VDW: 9.8056 Dipole/Dipole: -5.1817 Total Energy: 9.0026 kcal/mol |
|
Isomer B (axial)
Stretch: 1.5456 Bend: 5.1126 Stretch-Bend: 0.5536 Torsion: 8.7464 Non-1,4 VDW: -7.0755 1,4 VDW: 9.5848 Dipole/Dipole: -6.5008 Total Energy: 11.9668 kcal/mol |
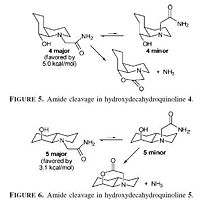
The results from molcecular modelling show that for both starting materials the equatorial form is lower in energy. Substituted cyclohexanes are known to favour the equatorial conformer as diaxial compressions are minimised.For the OH and peptide groups to react with one another they must be correctly orrientated (i.e. both axial or both equatorial). In the case of A this orienation is the lower energy conformer and hence will form the majority of the starting material, giving rapid hydrolysis of the peptide bond. However for B the necessary orienation is higher in energy and therefore provides an energy barrier which must be overcome before the reaction can take place, causing hydrolysis to take place more slowly at room temperature.
Regioselective Addition of Dichlorocarbene
Introduction

The diene pictured is reported[5] to react with electrophilic reaction selectively on the double bond exo to the chlorine atom. In this section computational modelling of the molecular orbitals and stretching frequencies of molecule are used to explain this selectivity.
Results and Discussion
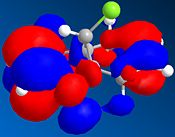
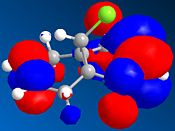
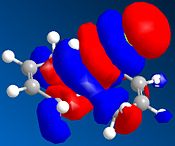
The table below shows a selection of the predicted molecular orbitals for the reactant diene.
| HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 |
|
|
|
|
|
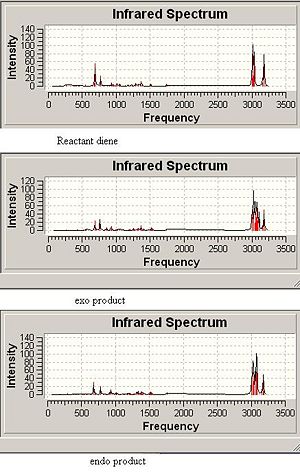
The C-Cl and C=C stretching frequencies for both the diene and both its exo and endo hydrogenated forms can be seen below.It can be seen that the C=C strectching frequencies remain fairly similar for both reactant and products. The C-Cl stretch however shows a noticeable differeance for the exo hydrogenated form yet the endo isomer ‘s stretch remains similar to the reactant. The C-Cl* orbital (LUMO+2) is known [6] to overlap with the exo π orbital (HOMO-1) and it can be seen from the predicted Mos that this is possible (same phase and necessary orienation for overlap). This would have the effect of lowering the overall energy of the molecule and also increasing the C-Cl bond length (donation into the antibonding orbital). As an increased bond length gives a higher wavenumber vibration, the exo product would be expected to have a lower frequency vibration which is inagreement with the computed result.
| reactant: | 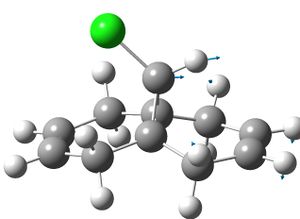 |
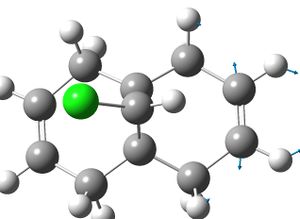 |
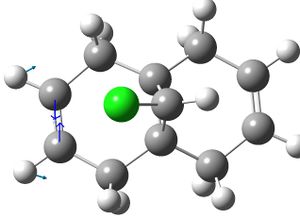 |
| exo hydrogenated product: | 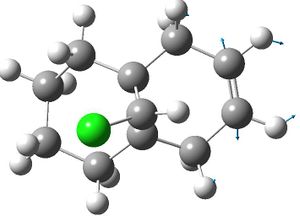 |
 |
| endo hydrogenated product: |  |
 |
Addition is more likely at the exo double bond due to it having a lower energy π orbital which is likely to be closer in energy to the LUMO of the attacking species.Fig 3 shows the computed spectra of all three isomers.
Mini project: Synthesis of Aziridino Epoxides from Cyclic Dienes
Introduction
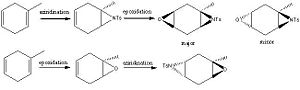
It has recenetly been reported[7] aziridino epoxides can be synthesised both regio and stereoselectively from cyclic dienes. The experimental method reported involves epoxidation using mCPBA and Sharpless aziridination (CAT, PTAB) but it is the order in which these two steps are carried out that determines the regiochemistry of the product. This section attempts to use computational modelling to predict the 13C NMR spectra for both regio isomers and to explain both the regio and stereoselectivity.
Prediction of 13C NMR Spectra
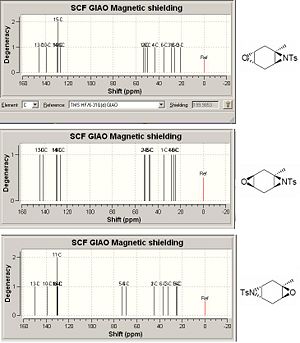
The regiosisomers may be destinguished using 13C NMR spectroscopy. The aziridine carbons are expected to to give peaks at about 40-50ppm in all products, yet the multiplets are expected to be different. The substituted epoxide isomer would be expected to give a doublet for the aziridine carbons as they are chemically equivalent whereas in the aziridine substituted isomer the carbons are no longer equivalent and would give a complex multiplet. This could also be done for the epoxide carbons however they are expected top be in a similar region to the methyl peaks and hence the aziridine is the better choice.
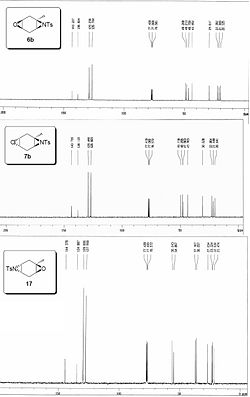
Computational models of the three products' spectra[8] were created using Gaussian (see fig5). The peaks above 120ppm represent the tosyl carbons and are not of interest. As expected the aziridine peaks show a doublet for the epoxidation/aziridination product and a complex multiplet for the aziridination/epoxidation isomers. This is in agreement with the experimental spectra (fig 6) however there is a noticeable difference in the chemical shifts.
 |
 |
Regioselectivity
The regiochemistry of the products is defined during the first step of the reaction, with both epoxidation and aziridination giving the most substituted product. The use of a simple MM2 model shows that epoxidation at the more substituted alkene gives a lower energy product (by approximately 17kcal/mol) as the ring is more distorted from planar reducing the steric clash between the epoxide and the ring hydrogens.
In contrast the model of the aziridination product shows that attack at the more substituted alkene is less energetically favourable due to the large steric contribution from the much bulkier tosyl group. However as the reaction is known to give the more substituted product, the regiochemistry of the product must be dependant upon the kinetics of the reaction and not the thermodynamics (as with epoxidation).
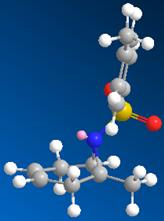
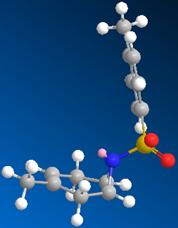
The product was obtained using the Sharpless aziridination method[11] the first step of which creates a bromonium ion intermediate that is then attacked by the tosyl amine (see fig 7). As the bromonium ion is closer in size to the epoxide oxygen than a tosyl group, it would reasonable to assume that a similar stabilsation to that of the substituted epoxide is experineced. Unfortunately ChemBio3D does not recognise the existance of a bromonium ion and as such this could not be explored.
Stereochemistry
The cyclic diene starting material is planar and as such has an equal probability of attack on either face in the first step of the synthesis. Therefore a racemic mixture of products would be expected. The overal reactants, though, show diastereoisomerism and it is the second step of the synthesis that defines this.
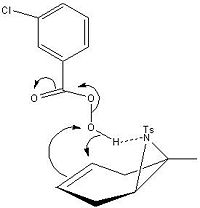
The MM2 models used in the regiochemistry section show that the aziridination/epoxidation products adopt a conformer that has the methyl group within the plane of the ring. It is therefore clear that the steric effect of te methyl is negligble compared to than of the epoxide/aziridine which both partially obstruct attack on one face of the molecule. However experimental result shows a 65:35 ratio in favour of a cis product when the aziridination is followed by epoxidation. Sureshkumar et al proposed a mechanism whereby the incoming mCPBA coordinates to the aziridine nitrogen (fig8) thereby directing the epoxide oxygen to the same face. Similar cases[12] [13]have been reported where mCPBA coordinates to an amine at C3, so it is likely that this is he case.
In contrast epoxidation followed by aziridation gives the trans stereoisomer. As the the mechanism for Sharpless epoxidation (fig 7) shows attack of the bromonium ion intermediate from the opposite face, it must be the formation of this intermediate that control the stereochemistry of the product. As the epoxide oxygen is electronegative and the bromonium ion carries a positive charge there would be a degree of electrostatic attraction between them which would cause the cis isomer intermediate to be more favourable. Attack on the opposite face by the tosyl amine then give the resulting trans product. Howener this could not be explored due to ChemBio3D not allowing a positive bromium.
References
- ↑ A. Radzickaand R.Wolfenden, J. Am. Chem. Soc. 1996, 118, 6105; DOI:10.1021/ja954077c
- ↑ J. W. Suggs and R.M.Pires, Tetrahedron Lett., 1997, 38, 2227; DOI:10.1016/S0040-4039(97)00330-4
- ↑ M. Fernandes, F. Fache, M. Rosen, P.-L. Nguyen, and D. E. Hansen, J. Org. Chem., 2008, 73, 6413; DOI:10.1021/jo800706y
- ↑ M. Fernandes, F. Fache, M. Rosen, P.-L. Nguyen, and D. E. Hansen, J. Org. Chem., 2008, 73, 6413; DOI:10.1021/jo800706y
- ↑ B.Halton and S. G. G.Russell, J. Org. Chem., 1991,56, 5553; DOI:10.1021/jo00019a015
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447; DOI:10.1039/P29920000447
- ↑ D.Sureshkumar, S. Maity, and S.Chandrasekaran J. Org. Chem, 2006, 71 (4), 1653; DOI:10.1021/jo052357x 10.1021/jo052357x
- ↑ Predicted NMR spectra can be found at http://hdl.handle.net/10042/to-1715, http://hdl.handle.net/10042/to-1716, http://hdl.handle.net/10042/to-1717
- ↑ D.Sureshkumar, S.Maity, and S.Chandrasekaran,J. Org. Chem., 2006, 71 (4),1653;DOI:10.1021/jo052357x
- ↑ J.Jeong, B.Tao, I.Sagasser, H.Henniges and K.B.Sharpless J. Am. Chem. Soc., 1998, 120 (27),6844;DOI:10.1021/ja981419g
- ↑ J.Jeong, B.Tao, I.Sagasser, H.Henniges and K.B.Sharpless J. Am. Chem. Soc., 1998, 120 (27),6844;DOI:10.1021/ja981419g
- ↑ P.O'Brien, A. C.Childs, G.J. Ensor, C.L.Hill, J.P.Kirby, M.J. Dearden, S.J.Oxenford, and C.M. Rosser Org. Lett., 2003, 5 (26), 4955;DOI:10.1021/ol035873n
- ↑ G. Asensio, R.Mello, C.Boix-Bernardini, M.E.Gonzalez-Nunez, and G.Castellano, J. Org. Chem., 1995, 60 (12), 3692;DOI:10.1021/jo00117a020