Rep:Mod:fmj172019
EX3 Section
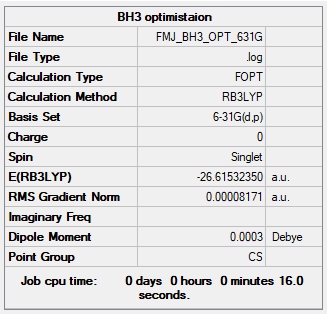
BH3
B3LYP/6-31G(d,p)
Ng611 (talk) 01:20, 15 May 2019 (BST) You should perform separate frequency and optimisation calculations. Also, I'm not sure why you've obtained a CS point group from this calculation.
Item Value Threshold Converged? Maximum Force 0.000203 0.000450 YES RMS Force 0.000098 0.000300 YES Maximum Displacement 0.000737 0.001800 YES RMS Displacement 0.000394 0.001200 YES Predicted change in Energy=-1.352798D-07
Frequency file: FMJ_BH3_SYM_FREQ.LOG
Low frequencies --- -0.2458 -0.1130 -0.0054 43.9715 45.1306 45.1313 Low frequencies --- 1163.6034 1213.5913 1213.5940
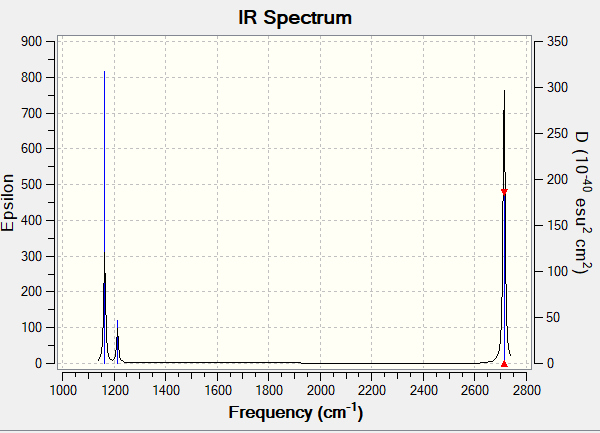
IR Spectrum
Vibrational spectrum for NH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 92 | A1 | yes | out-of-plane bend |
| 1214 | 14 | E | very slight | bend |
| 1214 | 14 | E | very slight | bend |
| 2580 | 0 | A1 | no | symmetric stretch |
| 2713 | 126 | E | no | asymmetric stretch |
| 2713 | 126 | E | no | asymmetric stretch |
Ng611 (talk) 01:18, 15 May 2019 (BST) Your 2x asymmetric stretches have a nonzero IR intensity and are therefore active. Also, you need to discuss why only three modes are present in your simulated spectrum.
BH3 |
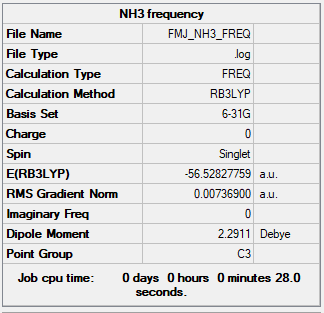
NH3
Ng611 (talk) 01:22, 15 May 2019 (BST) Where is your BH3 MO analysis?
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES Predicted change in Energy=-9.844596D-11 Optimization completed.
Frequency file: FMJ_NH3_FREQ.LOG
Low frequencies --- -337.0777 -179.7064 -179.7059 -0.0053 -0.0012 0.0171 Low frequencies --- 1072.4166 1741.0189 1741.0189
NH3 |
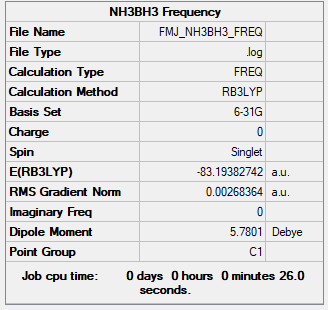
BH3NH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000122 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000513 0.001800 YES RMS Displacement 0.000296 0.001200 YES Predicted change in Energy=-1.631175D-07 Optimization completed.
Frequency file: FMJ_NH3BH3_FREQ.LOG
Low frequencies --- -179.4382 -70.6465 -66.4357 -0.0012 -0.0010 -0.0007 Low frequencies --- 207.6538 659.8771 661.2248
NH3BH3 |
Association energy= 2625.5[-(83.1938)-(-56.5283-26.6153)]=-32.8 kJ/mol
Ng611 (talk) 01:25, 15 May 2019 (BST) Your Bh3/BH3NH3 energies are correct, but your NH3 energy seems off. Did you double check your calculation? You also needed to include an appropriate bond enthalpy value from the literature to comment on the bond strength.
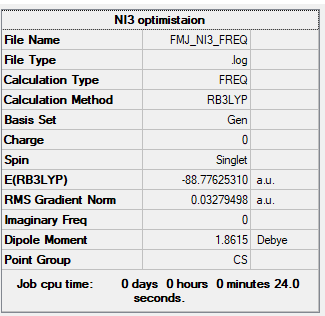
NI3
B3LYP/6-31G(d,p)LANL2DZ
Item Value Threshold Converged? Maximum Force 0.000061 0.000450 YES RMS Force 0.000037 0.000300 YES Maximum Displacement 0.000459 0.001800 YES RMS Displacement 0.000285 0.001200 YES Predicted change in Energy=-3.108653D-08 Optimization completed.
Frequency file: FMJ_NI3_FREQ.LOG
Low frequencies --- -62.7404 -62.5631 -61.1584 -0.0004 -0.0004 0.0001 Low frequencies --- 133.3070 134.7570 196.1498
NI3 |
The N-I bond length is 2.03 A.
Ng611 (talk) 01:27, 15 May 2019 (BST) You're off by a few tenths of an angstrom here...
Project Section
Isomers Assemble!
Ng611 (talk) 01:29, 15 May 2019 (BST) lol
Isomer 1:D2h Isomer 2:C2h Isomer 3:C2v Isomer 4:C2v Isomer 5:C1
Ng611 (talk) 01:29, 15 May 2019 (BST) Good assignment!
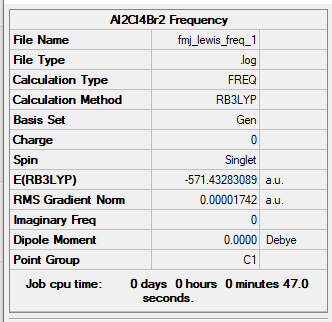
Isomer 1
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000053 0.000450 YES RMS Force 0.000024 0.000300 YES Maximum Displacement 0.001316 0.001800 YES RMS Displacement 0.000494 0.001200 YES Predicted change in Energy=-3.590082D-08 Optimization completed. ~~~~ You've applied a pseudopotential for both Cl and Br, when only Br should be subject to a pseudopotential.
Frequency file: FMJ_LEWIS_FREQ_1.LOG
Low frequencies --- -2.0033 -1.6013 0.0011 0.0014 0.0017 2.9567 Low frequencies --- 16.8954 62.3893 84.7666
Al2Br2Cl4:Isomer 1 |
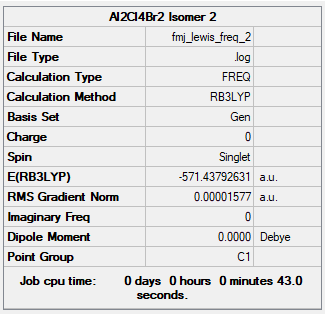
Isomer 2
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000044 0.000450 YES RMS Force 0.000015 0.000300 YES Maximum Displacement 0.000380 0.001800 YES RMS Displacement 0.000162 0.001200 YES Predicted change in Energy=-1.993027D-08 Optimization completed.
Frequency file: FMJ_LEWIS_FREQ_2.LOG
Low frequencies --- -3.2640 -1.6715 -0.0003 0.0005 0.0012 2.0341 Low frequencies --- 18.7855 47.6231 71.2520
Al2Br2Cl4:Isomer 2 |
The relative energy of the two isomers is 13.4 kJ/mol. Isomer 2 is more energetically stable than isomer 1 since the bromine atoms are further apart, decreasing the negative steric effect.
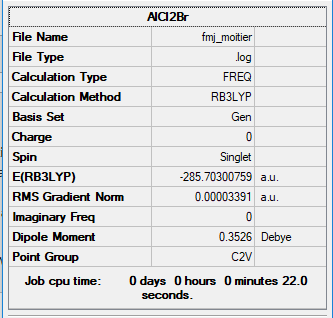
AlCl2Br
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000100 0.000450 YES RMS Force 0.000052 0.000300 YES Maximum Displacement 0.000547 0.001800 YES RMS Displacement 0.000361 0.001200 YES Predicted change in Energy=-4.145443D-08 Optimization completed.
Frequency file: FMJ_MOITIER.LOG
Low frequencies --- -0.0012 0.0006 0.0018 3.5705 5.9015 6.3396 Low frequencies --- 119.8512 132.8069 182.7664
AlCl2Br |
Dissociation Energy=2625.6[2(-285.7031)-(-571.4379)]= -83 kJ/mol Since dissociation energy is negative, formation of the monomers is energetically favourable, making them more stable than the dimer.
Ng611 (talk) 01:38, 15 May 2019 (BST) You've got the right number, but have the wrong sign (it should be positive, not negative). It looks like you made a slip plugging the numbers in as the sum you've given above is correct. Unfortunate that this has led you to the opposite conclusion.
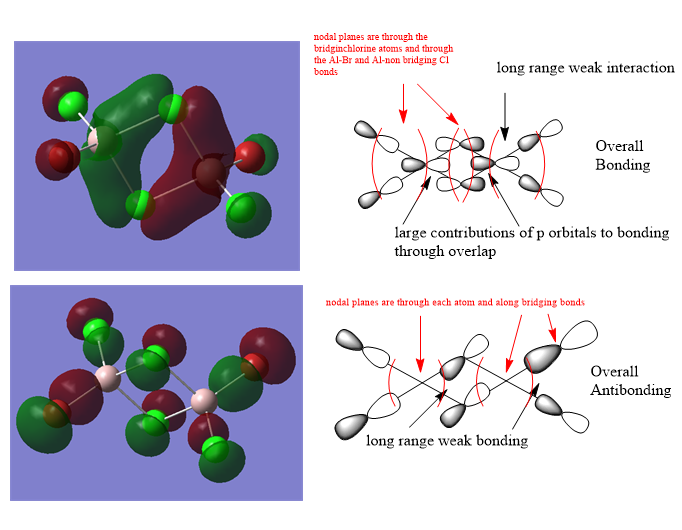
Molecular Orbitals of Isomer 2
Ng611 (talk) 01:40, 15 May 2019 (BST) In the second MO, the phase relationship of the terminal p orbitals don't correspond to the calculated MO diagram.