Rep:Mod:felicmod3
Mod 3- The Transition State
Here the Schrodinger equation is solved numerically using molecular orbital based method for the Transition state of large molecule. And the the TS are located using the shape of potential energy surface.
The Cope Rearrangement Tutorial
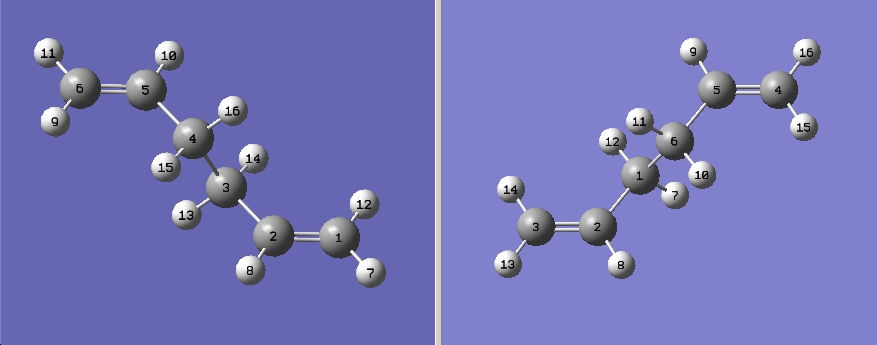
a) The anti form of 1,5- hexadiene is created and optimsed with (HF/3-21G) using Gaussian.
The energy for this structure is -231.69253506 a.u.
The point group is Ci
b) A 1,5-hexadiene with a gauche linkage is optimised like in part a (HF/3-21G), This form should be higher in energy than the anti form as the steric is less as the C=C are on both sides of the C-C bond, this make them further apart from each other.
 The energy for this structure is -231.69166702 a.u.
The energy for this structure is -231.69166702 a.u.
The point group is C1
Although it the gauche form is predicted to be higher in energy this is not true for this optimisation (5kcal/mol), this might due to the terminal C=C pi interaction. Or the method and basis set used is not accurate enough.
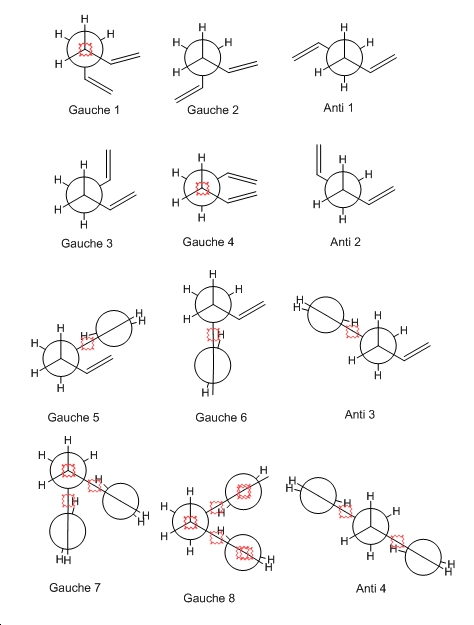
c) All possible form of the molecule is drawn below and optimised to determin which has the lowest energy.
d) All of the different forms optimised can be identified in appendix 1 with the same point group and similar energy. On my table there there are 2 pair of enantiomer of the gauche form.
e) The anti molecule optimised in part a) is the one with Ci point group.
f) Differnt method and basis set is compared here to compare the effect it make to the calculation and the structure.
The overall structure is the same(only small flatuations of bond length and dihedral angles for around 0.2A or 1degree) The struture optimsed using B3LYP/6-31G is more symmetric with slight longer bonds and wider angles.
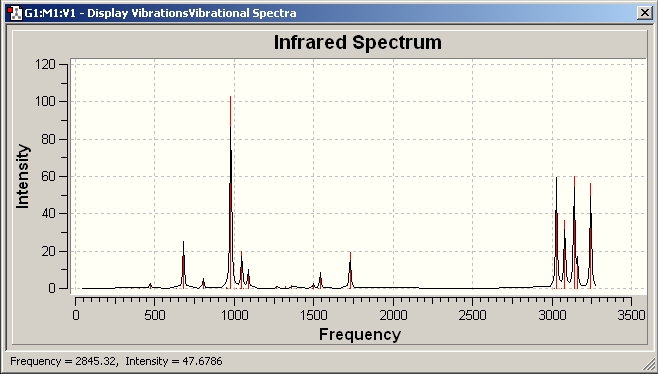
g) A frequency calculation is carried out to obtain the finnal energy with additinal term to compare.
IR spectrum for Ci Anti-conformoner
Additional terms can be obtained.
Sum of electronic and zero-point Energies= -234.416226
This is the PE at 0 K with zero pt vibrational energy
Sum of electronic and thermal Energies= -234.408944
Energy at 298.15 K and 1 atm with contribution of rotational vibrational and translational energy
Sum of electronic and thermal Enthalpies= -234.407999
This includes the correction for RT ( H=E+RT) improtant for dissociation reaction
Sum of electronic and thermal Free Energies= -234.447807
this includes the entropic contribution to free energy (G=H-TS)
Literature
- Brandon G. Rocque, Hason M. Gonzales and Henry F. Schaefer III , Molecular Physics, "2002", Vol. 100, 'No. 4', 441-446 DOI:10.1080/00268970110081412 2 10.1080/00268970110081412
"Boat" and "Chair" Transition Structure
In this section three different methods are used to optimise a transition structure
1. compute force constants on the beginning of calculation
2. Redundant coordinate editor is used
3. the QST2
a) a CH2CHCH2 fragment is drawn and optimised with HG/3-21G, 2 of this fragment is then used to create a transition state with the chair conformation with each end of the fragments are 2.2 Å apart
b) If the guessed transition state molecule has a reasonable geometry, it can be optimised by producing the -ve direction of the curvature and to compute its Hessian of the 1st step of opimisation.
The Hartee Fock method is used to optimised the chair TS created in part a) and its geometry is checked with the one in Appendix 2
An imaginary frequency of -818.045cm-1 is produced
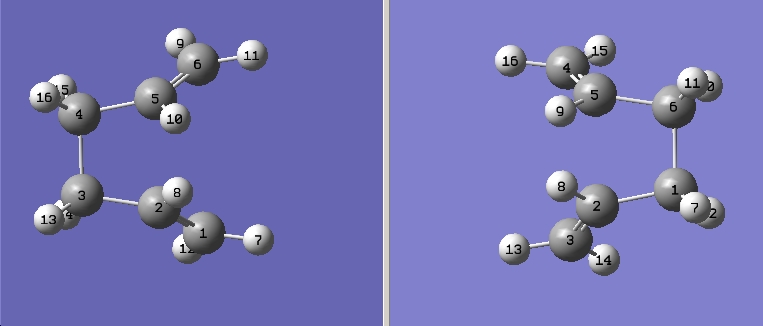
c) When the case that the guess structure does not quite match the actual geometry. Another method can be used to optimise the molecule, this is to freeze the reacting coordinate and minimise the rest of the molecule. After the molecule is relaxed, the reacting coorinate is 'un-frozen' and is optimised again.
The same guess structure is optimised using this method with 2 terminal C frozen.
[[image:]]
The 2 different method gives very similar structure although the bond distances is fixed to 2.2 Å for C)
d)
The two different TS optimisted by the two different method is quite differnt however the one optimised using the redundant coordinated method has optitmised to be very similar to the Gauche conformation of 1,5-hexadiene(C2 point group)
the bond forming/breaking lengths are quite different too, for the redundant corrdinate method is much longer (about 3 times)
e) Using the QST2 method has a lot of advantage, one of it is it's completely automated, and it can specify the product and reactants
Here the optimised Ci Anti 1,5-hexadiene is used, first the labelling of the molecule is changed so the product can corresponds to the reactant.
Than is it run with the QST2 method howere the job will fail.
It failed as the calculation method fail to locate the boat structure from reatant to product as it only interpolated between them linearly. ( not considering to rotate the allyl fragment around the central bond)
Therefore the geometry of the product and reactant has to be modified to be closer to the boat structure like below
f) From the previous exercise it is noticed that the boat conformation is similar to the Ci Anti conformer of 1,5-hexadiene while the chair TS is close to the C2 Gauche conformer of 1,5-hexadiene.
It cannot predict the reaction paths will lead to which conformer though
The IRC method takees small geometry stops to create a series of points with the gradient of when energy surface is steepest. Only the forward reaction direction is computed as the reaction coordinates are symmetrical. (force constant=once, no of pt= 50).
3 methods can be used to improve the calculation.
i) a normal minisation is ran using the last point on the IRC
ii) Specify a larger no of pt and restart IRC
iii) Run the IRC and specify that force constants is computed every step. (most accurate by most expensive and sometimes don't work on larger system)
The three approach is compared below.
g)
| Chair conformation | Boat Conformation | |
|---|---|---|
| Geometry | 
|

|
| Energy (a.u.) | -234.55863028 | -234.55927375 |
The Energy difference for both the Guache and Anti conformor for both calculation is about 1800kcal/mol
Literature
- Shogo Sakai, International Journal of Quantum Chemistry, "2000", Vol. 80, 1099–1106DOI:1099::AID-QUA59
Mini Project
In this Project we will look at the Diels Alder Cycloaddition, which is a type of pericyclic reaction. There is no intermediate form during this reaction as the bonds will be form/break in a concerted cyclic transition state. Wether the reaction is allowed or forbidden will be decided on the number of π orbital involved. Belows are a few rule
The reaction can only occur when the HOMO can interact with the LUMO. For the HOMO and LUMO to interact a significant overlapping between the orbitals are needed, so they need to be in the same symmetry.
Sometimes the dieneophine will have substituents with π bonds, these can interact with the product and stabilising the reaction.
For this reaction we are going to investigale it is a 4s +2s reaction as there are 4π from the butadiene and 2π from ethylene and the π orbital can be either a(asymmetric) or s (symmetric).
The LUMO of butadiene and HOMO of ethylene are s while the HOMO of butadiene and LUMO of ethylene are a. The same symmetry makes interaction possible between the HOMO and the LUMO.
i)The Reactant Molecule: Cis-butadiene
a cis-butadiene is created and optimised with HF/3-32G
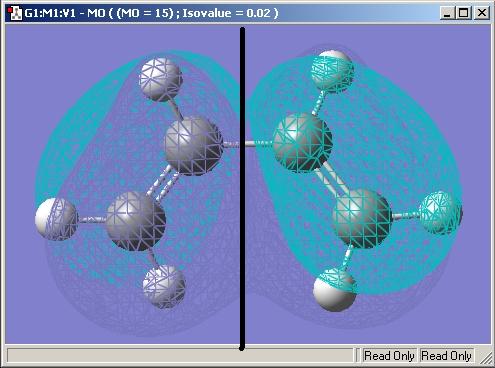
it's MO is then produced and the HOMO and LUMO is shown
HOMO : asymmetric
LUMO : symmetric
The LUMO is s and comfrims with the explanation above
ii)The Ethylene-Cis-butadiene Transition state
This TS has an envolopestructure that maximising the ethylene π orbital and π orbitals of butadiene overlapping. It is found from the lit that the interfragment distances is about 2.201Å.
The reaction coordinate is created using computed force constant matrix of the 1st step of the optimisation
| Bond | Bond Length (A) |
|---|---|
| C4=C3 | 1.36746 |
| C2=C1 | 1.36747 |
| C3-C2 | 1.39615 |
| C13=C11 | 1.22386 |
| C13--C4 | 2.19993 |
| C11--C1 | 2.20005 |
| Bond | Bond Angel (degree) |
| C4-C3-C2 | 121.585 |
| C1-C2-C3 | 121.630 |
| C11-C13-C4 | 111.407 |
| C3-C11-C1 | 111.399 |
| C13-C4-C3 | 100.701 |
| C11-C1-C2 | 100.674 |
Frequency
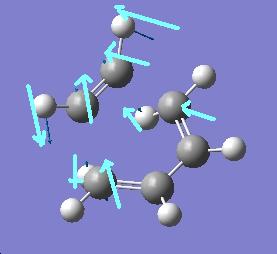
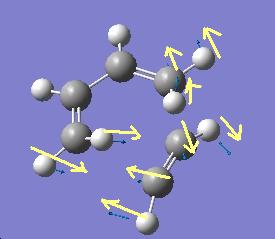
There is one negative frequency at -838.924cm-1 with the motion shown below
the lowest positive freqency is at 268.156cm-2 and the motion is shown below
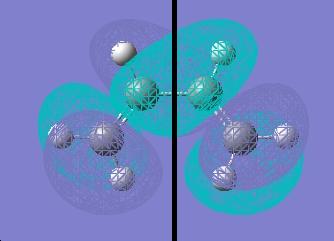
the MO diagram of ethylene-cis-butadiene for the HOMO and LUMO is shown below
HOMO: asymmetric (a)
LUMO: symmetric (s)
Literature
1. Shogo Sakai, J. Phys. Chem. A, "2000", 104 (5), 922-927 DOI:10.1021/jp9926894
2. Joey W. Storer, Laura Raimondi and K. N. Houk, J. Am. Chem. Soc., "1994", 116 (21), 9675-9683 DOI:10.1021/ja00100a037
3. Yi Li, and K. N. Houk, J. Am. Chem. Soc., "1993", 115 (16), 7478-7485 DOI:10.1021/ja00069a055
iii) The Cyclohexa-1,3-diene reaction with Maleic Anhydride
Maleic anhydride and cyclohexa-1,3-diene undergo facile Diels-Alder to give a endo product. This is the kinetic prouct as the exo TS has high energy. Here the regioselectivity is studied
The endo and exo structure are optimised and thier structure is optimised and studied. A guess structure is 1st created, as the guess structure is quite inaccurate it is optimised by freezing the reaction co-ordinate and then the rest of the molecule will be minised, after this the reacting coordinate is unfrozen and fully relaxed and is optimised again.
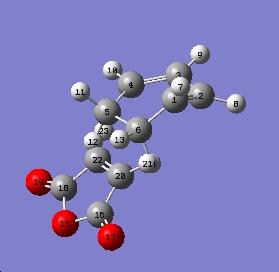
Optimised endo TS
| Bond | Bond Length (A) |
|---|---|
| C4..C20 | 1.54725 |
| C1..C22 | 1.54000 |
| C6-C5 | 1.53184 |
| C2-C3 | 1.550425 |
| C3=C4 | 1.54011 |
| C1=C2 | 1.54202 |
| C22=C20 | 1.53268 |
| C22-C18 | 1.51702 |
| C20-C16 | 1.51604 |
| C18-O15 | 1.38328 |
| C16-C15 | 1.39284 |
| C18=O19 | 1.89283 |
| C16=O17 | 1.89328 |
Optimised exo TS
| Bond | Bond Length (A) |
|---|---|
| C1..C20 | 1.54726 |
| C4..C22 | 1.54090 |
| C6-C5 | 1.55127 |
| C2-C3 | 1.56034 |
| C3=C4 | 1.54341 |
| C1=C2 | 1.54212 |
| C22=C20 | 1.53227 |
| C22-C18 | 1.51969 |
| C20-C16 | 1.51958 |
| C18-O15 | 1.38953 |
| C16-C15 | 1.39661 |
| C18=O19 | 1.89732 |
| C16=O17 | 1.89732 |
Both TS has similar bond lengthes
Transtion state energies (a.u.)
Endo = -606.90823898 Exo = -606.90823902
The exo have slighly higner energy so the endo is slightly more thermodynamically stable
MO diagram
Endo HOMO
Asymmetric
Endo LUMO
Asymmetric
Exo HOMO
Asymmetric
Endo LUMO
Asymmetric
The endo form is decided to be thernodynamically more stable as the bonding interaction secondary orbital interaction from the C=O groups interacts with the C=C of the cis butadiene and create a good overlap. This is abscense in the Exo case as they are not in the same orientation.