Rep:Mod:fatt06
The hydrogenation of cyclopentadiene dimer

Cyclopentadiene dimerises via a Diels Alder reaction to produce the endo dimer 2, rather than the exo dimer. Diels Alder reactions are thermal [2+4] cycloadditions, whose mechanism involves the sigma overlap of the pi orbitals. The HOMO of the diene interacts with the LUMO of the dienophile. Diels Alder reactions have endo selectivity. The endo product is usually favoured by kinetic control due to secondary orbital effects. Endo attack leads to the more stable transition state. The exo dimer (133.4 kJ/mol) is lower in energy than the endo dimer (142.3 kJ/mol).
This shows that the endo dimer is the kinetic product and the exo dimer is the thermodynamic product. The endo product can be hydrogenated to give one of the dihydro products 3 or 4. From the energy calculation obtained using MM2 force field, product 4 (130.4kJ/mol) is more stable than product 3 (150.3kJ/mol).
| Type of energy | Product 3 | Product 4 |
|---|---|---|
| Stretch | 5.23 | 4.59 |
| Bend | 79.1 | 60.75 |
| Torsion | 50.6 | 52.3 |
| Van der Waals | 24.0 | 18.8 |
| H-bonding | -6.28 | -4.45 |
Product 4 is the thermodynamically more stable product compared to product 3, this means that the endo alkene (with respect to the bridge), is easier to hydrogenate compared to the exo alkene. The endo alkene is more hindered than the exo alkene due to the bridging methyl group. A double bond reduces the C-C bond length, this induces more strain in the ring containing the methyl bridge, therefore hydrogenation of the endo alkene relieves strain in the ring and is more favourable. Product 4 is the thermodynamic product as it is more stable and product 3 is the kinetic product as hydrogenation takes place at the least strained/ hindered alkene to give a higher energy hydrogenation product. The bending energy has the hugest contribution to the energy difference between the two hydrogenation products (19.4 kJ/mol).
Stereochemistry of nucleophilic addtions to a pyridinium ring (NAD+ analogue)
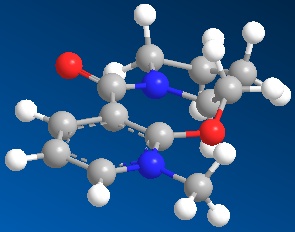
The prolinol derivative (5) has anenergy of 240.33kJ/mol. In the prolinol derivative, the carbonyl group is orientated on top relative to the pyridinium ring. The carbonyl group is about 20 degrees above the plane of the ring. This explains why when it reacts with the Grignard, the methyl is added to the top face of the pyridinium ring. The carbonyl oxygen interacts with the magnesium of the Grignard reagent; therefore the methyl is added to the same face as the oxygen.
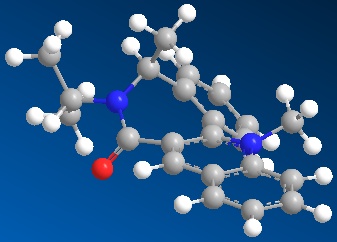
The pyridinium derivative (7) has an energy of 411.9kJ/mol. The energy is higher compared to the prolinol derivative (5) due to the larger number of atoms and bonds. The carbonyl group is situated about 38 degrees below the plane of pyridinium ring. This explains why a nucloephile (aniline) would add to the top face of the aromatic ring. In the case of aniline as the nucleophile, approach from a face opposite to the carbonyl avoids steric repulsion between the carbonyl oxygen and the nitrogen and also between the carbonyl group and the phenyl ring.
The geometry can be further improved by using the dft method for geometry optimization. The density functional theory (dft) is a quantum mechanical theory used to investigate the electronic structure (the ground state) of molecules.
Stereochemistry and reactivity of an intermediate in the synthesis of Taxol
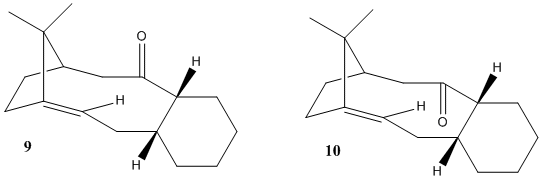
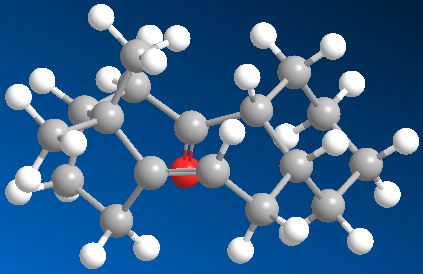
The intermediate in the synthesis of taxol can have the carbonyl group pointing either up (9) or down(10). Isomer 10, with the carbonyl pointing down is more stable than isomer 9. In intermediate 9, the fused cyclohexane ring adopts a twist boat conformation and a chair conformation in intermediate 10. In isomer 9, there is an unfavourable steric interaction between the carbonyl group and the isopropyl bridge, therefore the carbonyl group does not point straight upwards, but tilts slightly to the outside of the ring.
The alkene can be thought to react slowly due to huge steric clashes of the approaching reagent on both the faces of the alkene. The alkene is highly substituted which means that it is highly stable. On the top face the isopropyl bridge hinders the approach of reagents. On the bottom face the cis geometry of the alkene substituents hinders the approach of reagents. In isomer 9, the carbonyl group further hinders the approach of any nucleophiles from the top face. In isomer 10 the carbonyl group hinders approach of nucleophiles from the bottom face.
The olefin strain energy is defined as the difference in energy between the strain energy of an olefin and that of its parent hydrocarbon and can be used to predict the stability and reactivity of bridgehead olefins. The lower the olefin strain energy the more stable the bridgehead alkene is. Hyperstable olefins contain less strain than the parent hydrocarbon and thus have negative olefin strain energies. These olefins are unreactive, not due to steric hindrance or the enhanced pi-bond strength, but due to the greater strain of the parent hydrocarbon.
Kinetic factor for slow reactivity: steric hindrance about the double bond Thermodynamic factor for slow reactivity: the olefin is more stable than the parent cycloalkane, therefore hydrogenation is not favoured.
Using the MMFF94 field, isomer 10 is still more stable than isomer 9. However, both isomers are higher in energy using the MMFF94 force field compared to the MM2 force field.
Regioselective addition of dichlorocarbene
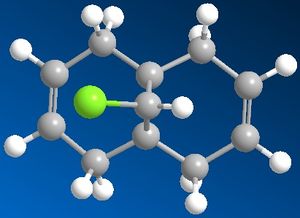


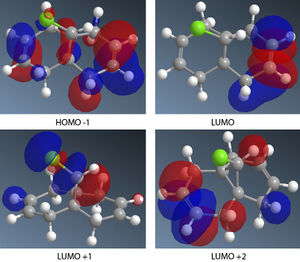
Using the MM2 force field the lowest energy conformation has an energy of 74.9kJ/mol. The MOPAC/PM6 molecular orbital method was used to provide an approximate representation of the valence electron molecular wavefunctions. In the HOMO, the pi bonding electron cloud lies mainly on the endo alkene, the electron cloud is mainly on the endo ring. This shows that more electron density is on the endo alkene thus making it more nucleophilic than the exo alkene. Reaction with dichlorocarbene is similar to an electrophilic addition and will be selective for the endo alkene.
| Bond | Dialkene | Monoalkene |
|---|---|---|
| C=Cexo | 1737.11 | |
| C=Cendo | 1757.37 | 1758.05 |
| C-Cl | 770.91 | 774.95 |
The exo double bond stretching frequency is weaker than that of the endo double bond. This is because the exo double bond donates electron density into the C-Cl sigma antibonding orbital, thus weakening it. The monoalkene endo double bond (C=C) has a slightly higher stretching frequency than that of the dialkene. This means that the monoalkene double bond is stronger than the dialkene endo C=C bond. The C-Cl stretching frequency in the monoalkene is higher than that of the dialkene. The difference in strength of the C-Cl bonds can be explained by looking at the involvement of the exo alkene in the dialkene. The LUMO+1 molecular orbital is mainly a sigma antibonding orbital on the C-Cl bond. The HOMO-1 shows a large pi electron cloud on the exo alkene. The exo alkene pi bond donates electron density into the C-Cl antibonding orbital, thus weakening the C-Cl bond. In the mono-hydrogenated derivative, there is no donation into the C-Cl antibonding, thus it is stronger and has a higher stretching frequency.
Structure based Mini project using DFT-based molecular orbital methods
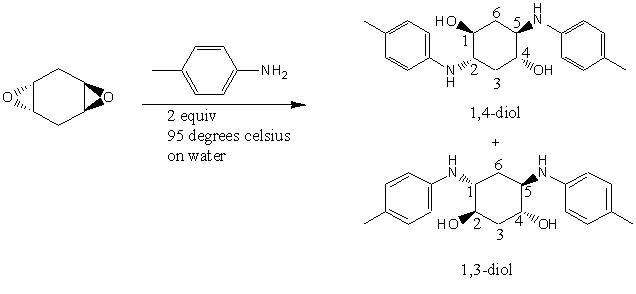
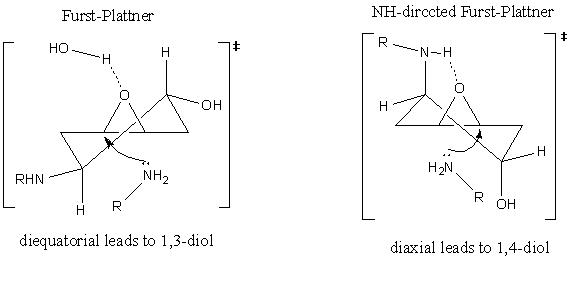
The mini project chosen was that of the Regioselective synthesis of aniline-derived 1,3- and Ci-symmetric 1,4-diols from trans-1,4,-cyclohexadiene dioxide. This reaction is an example of epoxide ring openingDOI:10.1021/ol902856b . The reaction is known to give 1.3 diol products in high selectivity due to Furst-Plattner(trans diaxial effect) control of ring opening. in the mechanis, the first step involves the ring opening of one epoxide. The intermediate can nave two conformations, one with the amino and hydroxyl groups diaxial and the more stable one with the groups diequatorial. In the diaxial conformer, the NH will hydrogen bond to the epoxide oxygen (NH directed Furst-Plattner), thus activating it for attack and giving the 1,4 diol. in the diequatorial conformer, the epoxide is activated by hydrogen bonding to the to water and this gives the 1,3 diol product. Both mechanisms go via chair- like transition states.
Sketches of the molecules were drwan using ChemBio 3D. The MMM2 force field was used to refine the geometries of the molecules. the 1,4-diol had an energy of 0.233kJ/mol and the 1,3-diol had an energy of 32.23kJ/mol. The geometry was then optimized using the DFT/MPW1PW91 method and the basis set was set to 6-31G(d,p). the file was submitted to the SCAN for geometry optimization, resultant otpimized geometry file was submitted to SCAN for NMR chemical shift calculation.
The carbon 13 NMR spectra for the two isomers were predicted. The important chemical shifts are those of the cyclohexane ring as theses help to distinguish between the two isomers. The chemical shifts for the amino benzyl substituents will be similar in both molecules but will not help in distinguishing between the isomers.
| Carbon | Predicted | Literature | Difference |
|---|---|---|---|
| C6 | 31.2 | 31.6 | 0.4 |
| C3 | 37.3 | 36.9 | 0.4 |
| C1 | 54.1 | 54.9 | 0.8 |
| C5 | 54.5 | 54.9 | 0.5 |
| C2 | 68.3 | 70.6 | 1.2 |
| C4 | 68.4 | 70.6 | 1.3 |
| Carbon | Predicted | Literature | Difference |
|---|---|---|---|
| C3 | 36.2 | 36.5 | 0.3 |
| C6 | 36.3 | 36.5 | 0.2 |
| C1 | 63.9 | 56.2 | 7.7 |
| C5 | 64.0 | 56.2 | 8.2 |
| C2 | 72.6 | 71.2 | 1.4 |
| C4 | 72.7 | 71.2 | 1.5 |
The predicted carbon 13 NMR chemical shifts agree with those provided in the literature. There is a small difference in the chemical shifts ranging from 0.2-1.5ppm. The largest difference in chemical shift is observed for carbons 1 and 5 of the 1,4-diol, where the difference is 7-8ppm. In the 1,3-diol,DOI:10042/to-3733 carbons 2 and 4 (attached to the hydroxyl groups)resonate at 70.6ppm and carbons 1 and 5 (attached to the amino groups) resonate at 54.9ppm. The carbons resonate at the same chemical shift because they have the same chemical and magnetic environments. Carbons 2 and 4 have higher chemical shifts because they are deshielded by the highly electronegative oxygen atoms. Nitrogen has a smaller electronegativity compared to oxygen, therefore the C5 and C1 chemical shifts are lower. Carbons 3 and 6 have different chemical shifts as they are in different environments. C3 has a higher chemical shift than C6 as it is deshielded by the oxygen atoms two bonds away. The NMR spectrum of the 1,3diol has four different chemical shifts for the cyclohexane carbons. The NMR spectrum of the 1,4diol DOI:10042/to-3734 has three different chemical shifts for the cyclohexane carbons. C1 and C4 (71.2ppm) have high chemical shifts due to the deshielding effect of the oxygen. C2 and C5 (attached to amino group) have a chemical shift of 56.2 and C3 and C6 have a chemical shift of 36.5ppm. Tyhe two isomers can be distinguished form one another in that the 1,3 diol will have 4 different resonances for the cyclohexane carbons and the 1,4 diol will only have 3 different chemical shifts for the cyclohexane.
