Rep:Mod:et2118
NH3
Summary Information
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy E(RB3LYP) | -56.55776873 au |
| RMS gradient | 0.00000485 au |
| Point group | C3v |
| Optimised bond distance | 1.02Å |
| Optimised bond angle | 106° |
| NBO charge on N | -1.125 |
| NBO charge on H | 0.375 |
Item table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
3D Jmol
NH3 |
The optimisation file is linked here
Vibrations in the IR spectrum

| Wavenumber (cm-1) | Symmetry | Intensity |
|---|---|---|
| 1090 | A1 | 145 |
| 1694 | E | 14 |
| 1694 | E | 14 |
| 3461 | A1 | 1 |
| 3590 | E | 0 |
| 3590 | E | 0 |
From the 3N-6 rule, there should be 6 vibrational modes. The vibrations at 1694cm-1 and at 3590cm-1 are degenerate and have the same energy. The vibrational modes at 1090cm-1 and 1694cm-1 are bending vibrations. The vibrations at 3461cm-1 and 3590cm-1 are bond stretch vibrations. The vibrations at 1090cm-1 and 3461cm-1 are both highly symmetric. The vibrational mode at 1090cm-1 is known as the umbrella mode. In an experimental spectrum of gaseous ammonia, one would expect to see three bands.
The charge is expected to be negative for N and positive for H because nitrogen is more electronegative than hydrogen and would withdraw electron density more, forming a partial negative charge on the nitrogen.
N2
Summary Information
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy E(RB3LYP) | -109.52412868 au |
| RMS gradient | 0.00000365 au |
| Point group | D∞h |
| Optimised bond distance | 1.11Å |
| Optimised bond angle | N/A |
| NBO charge on N | 0.000 |
Item Table
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000002 0.001800 YES RMS Displacement 0.000003 0.001200 YES
3D Jmol
N2 |
The optimisation file is linked here
Vibrations in the IR spectrum
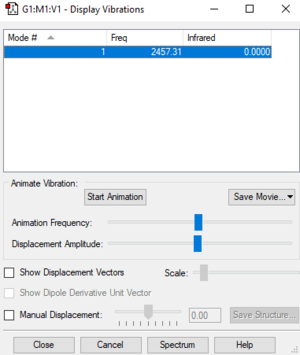
| Wavenumber (cm-1) | Symmetry | Intensity |
|---|---|---|
| 2457 | SGG | 0 |
There is one vibrational mode that would not appear in the IR spectrum because there is no change in dipole moment.
H2
Summary Information
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy E(RB3LYP) | -1.17853936 au |
| RMS gradient | 0.00000017 au |
| Point group | D∞h |
| Optimised bond distance | 0.74Å |
| Optimised bond angle | N/A |
| NBO charge on H | 0.000 |
Item table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
3D Jmol
H2 |
The optimisation file is linked here
Vibrations in the IR spectrum
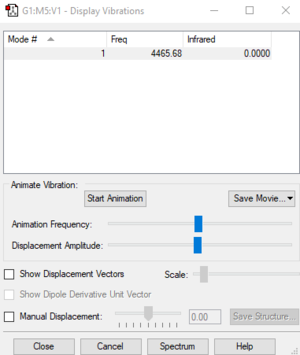
| Wavenumber (cm-1) | Symmetry | Intensity |
|---|---|---|
| 4466 | SGG | 0 |
There is one vibrational mode that would not appear in the IR spectrum because there is no change in dipole moment.
Conquest
[CAMVOB ]
The N-N bond distance in the mono-metallic transition metal complex, bis(Dinitrogen)-bis(1,2-bis(dimethylphosphino)ethane)-chromium, is 0.9843Å compared to 1.11Å computationally. The N-N bond distance in the transition metal complex is shorter than the computational bond distance both due to the program Gaussian calculating bond distances to an accuracy of 0.01Å and the difference in distribution of electron density only around the N-N bond computationally compared to in the transition metal complex. In the transition metal complex, some of the electron density is used to form the bond between the transition metal and the nitrogen. Less electron density around the bond makes the bond weaker and longer.
Energy of the Haber-Bosch process
E(NH3) = -56.55776873 au
2*E(NH3) = -113.1155375 au
E(N2) = -109.52412868 au
E(H2) = -1.17853930 au
3*(E(H2)= -3.5356179 au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -146.5 KJ/mol
The energy needed to convert hydrogen and nitrogen gas into ammonia gas is -146.5 KJ/mol. The ammonia product is more stable.
CO
Summary Information
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy E(RB3LYP) | -113.30945314 au |
| RMS gradient | 0.00000433 au |
| Point group | C∞v |
| Optimised bond distance | 1.14Å |
| Optimised bond angle | N/A |
| NBO charge on C | 0.506 |
| NBO charge on O | -0.506 |
Item table
Item Value Threshold Converged? Maximum Force 0.000007 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000003 0.001800 YES RMS Displacement 0.000004 0.001200 YES
3D Jmol
CO |
The optimisation file is linked here
Vibrations in the IR spectrum
| Wavenumber (cm-1) | Symmetry | Intensity |
|---|---|---|
| 2209 | SG | 68 |
There is one vibrational mode that would appear in the IR spectrum.
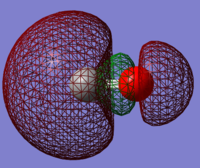
Molecular Orbitals
This molecular orbital is the deepest in energy at -19.25806 au. The occupied 1s atomic orbital on the oxygen atom contributes to the molecular orbital. The 1s atomic orbital is held tightly to the oxygen atom therefore it is not very involved with chemical bonding. This is a core occupied non bonding orbital.
This occupied bonding molecular orbital is made up of mainly the 2s orbital on carbon and 2p orbital on oxygen. This molecular orbital is somewhat deep in energy.
The py orbitals on both atoms overlap to form a pi bond, resulting in electron density both above and below the atoms, with a node in between, forming an occupied bonding molecular orbital.
This molecular orbital is the highest occupied molecular orbital, made up of the 2pz orbital of the oxygen atom interacting with the 2p orbital of the carbon atom, seen through the nodes on both atoms. This is an occupied bonding molecular orbital.
This is the lowest unoccupied molecular orbital which is high in energy and does not contribute to bonding. The molecular orbital is formed from two out of phase p orbitals on the carbon and oxygen atoms, forming an antibonding molecular orbital. The difference in size of the orbitals is due to the electronegativity of the oxygen atom, making the p orbitals on the oxygen smaller.
CH4
Summary Information
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy E(RB3LYP) | -40.52401404 au |
| RMS gradient | 0.00003263 au |
| Point group | Td |
| Optimised bond distance | 1.09Å |
| Optimised bond angle | 109° |
| NBO charge on C | -0.930 |
| NBO charge on H | 0.233 |
Item table
Item Value Threshold Converged? Maximum Force 0.000063 0.000450 YES RMS Force 0.000034 0.000300 YES Maximum Displacement 0.000179 0.001800 YES RMS Displacement 0.000095 0.001200 YES
3D Jmol
CH4 |
The optimisation file is linked here
Vibrations in the IR spectrum
| Wavenumber (cm-1) | Symmetry | Intensity |
|---|---|---|
| 1356 | T2 | 14 |
| 1356 | T2 | 14 |
| 1356 | T2 | 14 |
| 1579 | E | 0 |
| 1579 | E | 0 |
| 3046 | A1 | 0 |
| 3162 | T2 | 25 |
| 3162 | T2 | 25 |
| 3162 | T2 | 25 |
One would expect to see 2 bands in the IR spectrum.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 0.5/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - You correctly described two sets of degenerate modes which explains a spectrum with 4 bands. The intensities of all stretching vibrations are too low to be observed in an experimental spectrum, so only two bands are expected.
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES - your explanation of the second displayed MO being a combination of 2s orbitals would result in an anti-bonding-orbital rather than a bonding one as you stated. You missed to explain the relative order in energy (e.g. based on the number of nodes).
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
YES- however, you could have analysed these result in more detail rather than only stating the calculated information.
Do some deeper analysis on your results so far