Rep:Mod:emw15page
IMM2 Wiki page
NH3 Molecule
The optimisation file is liked to here
What is the molecule? NH3 (Ammonia)
What is the calculation method? RB3LYP
What is the basis set? 6-31G(d,p)
What is the final energy E(RB3LYP) in atomic units (au)? -56.55776873 a.u.
What is the RMS gradient? 0.00000485 a.u.
What is the point group of your molecule? C3V
N-H bond distance: 1.01798 angstrom
Optimised H-N-H bond angle: 105.741 degrees
Item table for NH3
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
test molecule |
The optimisation file is liked to here
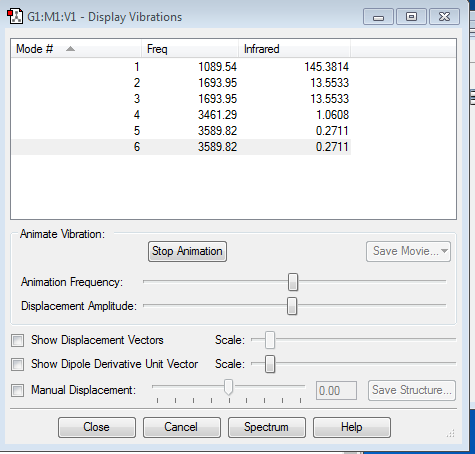
NH3 Vibrations and Charges
how many modes do you expect from the 3N-6 rule?
since N=4, 6 modes are expected
which modes are degenerate (ie have the same energy)?
modes 2&3 and 5&6 are degenerate, as they have the same frequency and intensity of absorption.
which modes are "bending" vibrations and which are "bond stretch" vibrations?
modes 1,2&3 are bending while modes 4,5&6 are stretching
which mode is highly symmetric?
modes 1 and 4 are highly symmetric
one mode is known as the "umbrella" mode, which one is this?
the first mode is known as the umbrella mode
how many bands would you expect to see in an experimental spectrum of gaseous ammonia?
There should be 4 distinctly observable absorption bands as although there are 6 modes, there are 2 pairs of degenerate modes which would show up as 2 different bands instead of 4.

charge on Nitrogen: -1.125
charge on Hydrogen: 0.375
It would be expected that the charge on the nitrogen would be more negative than the hydrogen as it is more electronegative. the slight positive charge on the hydrogen would be expected since it only has one electron which would be used in bonding with the electronegative nitrogen which would pull the electron density towards itself, leaving the hydrogen atom with a slight positive charge.
Haber-Bosch Energy calculations
N2
The optimisation file is liked to here

Summary information:
N-N optimised bond length: 1.10550 angstrom.
Note that N2 has a triple bond and so is a linear molecule. Also, it does not have a point group symmetry as it is only a diatomic molecule.
Calculation method: RB3LYP
Basis Set: 6-31G(d,p)
RMS Gradient: 0.00000060 a.u.
Final Energy: -109.52412868 a.u.
N2 item table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
H2
The optimisation file is liked to here

Summary information:
H-H optimised bond length: 0.60000 angstrom.
H2 is a homodinuclear molecule and so has a linear bond. Also, it doesnot have a point group symmetry as it is only a diatomic molecule
Calculation method: RB3LYP
Basis Set: 6-31G(d,p)
RMS Gradient: 0.00000017 a.u.
Final Energy: -1.17853936 a.u.
H2 item table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Energy for the Haber-Bosch Process
E(NH3)= -56.55776873 a.u.
2*E(NH3)= 113.115537 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= 3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579092 a.u. =-146.47906 kJ/mol
Note to calculate from Hatrees(a.u.) to kJ/mol, we multiply by 2625.5.
The energy for converting hydrogen and nitrogen gas into ammonia gas is -146.47906 kJ/mol. Since the reaction is exothermic, the ammonia product is more stable than the hydrogen and nitrogen reactants. Compared to literature values for the Haber-Bosch reaction, the enthalpy change for the reaction should be around -45.64 kJ/mol[1]. The large deviation from the theoretically calculated value to the literature value is largely due to the fact that the literature value is an experimentally determined value. The theoretically calculated value assumes that the process is 100% efficient and there is no heat loss to the environment while in reality, this is far from the case.
Molecular Orbitals
Below are some examples of molecular orbitals that were modeled for N2 using the Gaussian Software.





Project Molecule: Chlorine Trifluoride
The optimisation file is liked to here
Chlorine Trifluoride |
Cl-F axial bond length: 1.72932 angstrom
Cl-F equitorial bond length: 1.65025
F-Cl-F bond angle: 87.161 and 174.322 degrees
Calculation method: RB3LYP
Basis Set: 6-31G(d,p)
RMS Gradient: 0.00021787 a.u.
Final Energy: -759.46531599 a.u.
Symmetry: C2v
Item Table for ClF3
Item Value Threshold Converged? Maximum Force 0.000440 0.000450 YES RMS Force 0.000212 0.000300 YES Maximum Displacement 0.001550 0.001800 YES RMS Displacement 0.000934 0.001200 YES
Background on Chlorine Trifluoride
The T-shaped geometry of chlorine trifluoride is in accordance with the VSEPR theory which would suggest a distorted trigonal bipyramidal structure[2] - since the central atom is surrounded by 3 bonding pairs and 2 lone pairs of electrons. Note that the eqitorial and axial bonding pairs are not energetically equivalent due to difference in orientation and bond lengths.
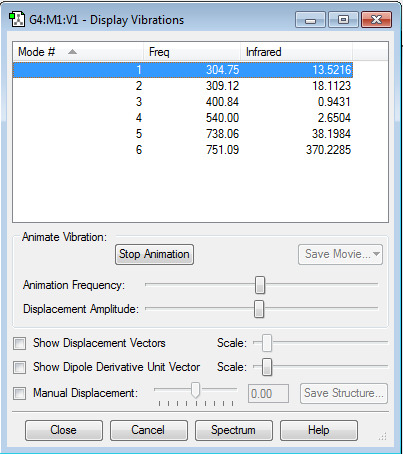
Vibrations and charges
how many modes do you expect from the 3N-6 rule?
since N=4, 6 modes are expected
which modes are degenerate (ie have the same energy)?
There are no degenerate modes as the frequency and intensity of absorption for each mode are all differing.
which modes are "bending" vibrations and which are "bond stretch" vibrations? modes
modes 1,2 and 3 are bending modes 4.5 and 6 are stretching
which mode is highly symmetric?
mode 4 is highly symmetric
one mode is known as the "umbrella" mode, which one is this?
mode 2 is the umbrella mode
how many bands would you expect to see in an experimental spectrum of gaseous Chlorine Trifluoride?
6 distinct bands will be expected since there are 6 non-degenerate modes

Charge on Chlorine: 1.225
Charge on axial Fluorine: -0.454
Charge on equatorial Fluorine: -0.316
The negative charges on the fluorines are as expected due to the higher electronegativity of fluorine compared to chlorine - the fluorine atoms have a stronger ability to pull electron density towards themselves, resulting in this negative charge while leaving chlorine with a positive charge since the overall charge of the molecule should be zero. It also follows accordingly that the axial fluorines have a more negative charge compared to the equatorial fluorine due to their geometry and shorter bond distance from the chlorine, it can pull more electron density than the equatorial fluorine.
Molecular Orbitals
Below are some examples of molecular orbitals that were modeled for ClF3 using the Gaussian Software.





References
(1) Frattini D, Cinti G, Bidini G, Desideri U, Cioffi R, Jannelli E. A system approach in energy evaluation of different renewable energies sources integration in ammonia production plants. Renewable Energy [Internet]. 2016 [cited 10 March 2017];99:472-482. Available from: http://www.sciencedirect.com/science/article/pii/S096014811630636X
(2) Müller H. The rotational spectrum of chlorine trifluoride, ClF3. Centrifugal distortion analysis, Cl nuclear magnetic shielding tensor, structure, and the harmonic force field. Physical Chemistry Chemical Physics [Internet]. 2001 [cited 10 March 2017];3(9):1570-1575. Available from: http://pubs.rsc.org/en/content/articlehtml/2001/cp/b100527h