Rep:Mod:einstein
James Cochrane's Wiki
H + H2 H + H2
In the following analysis of the collision between an H atom and an H2 molecule, the atoms are constrained to be collinear (collision angle is 180). It is important to realize that although the potential barrier is least for collinear attack, other lines of attack are feasible and contribute to the overall rate of reaction in reality. Additionally, effects of quantum mechanical tunnelling on reactivity are ignored; we just consider the classical trajectories of particles over surfaces.[1]
Two parameters are required to define the nuclear separation: the HA-HB separation and the HB-HC separation . A plot of the total energy of the system against and gives the potential energy surface of this reaction.
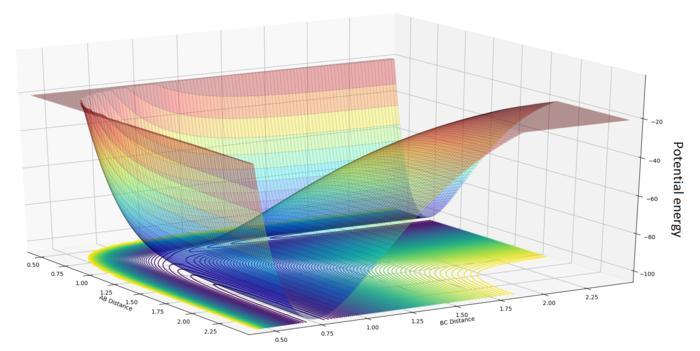
Although the 3D surface provides a useful visualization tool, contour diagrams (with contours of equal potential energy) will be more suitable for analysis.
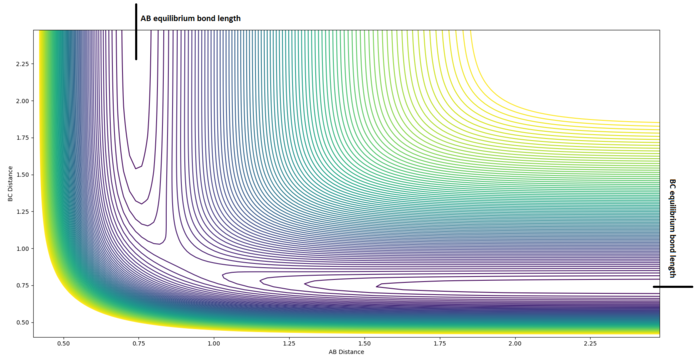
Here, I use the notation , and , where is the potential energy function.
Suppose that has a local extremum at a point , then . These properties hold true at both a transition state and at minimum. To distinguish between these two structures we must use the following formula: . If and , we are at a minimum. Whereas, if and , we are at a transition state. Additionally, if , we are at a saddle point.[2]
(Perfect! Fjs113 (talk) 17:51, 14 May 2018 (BST))
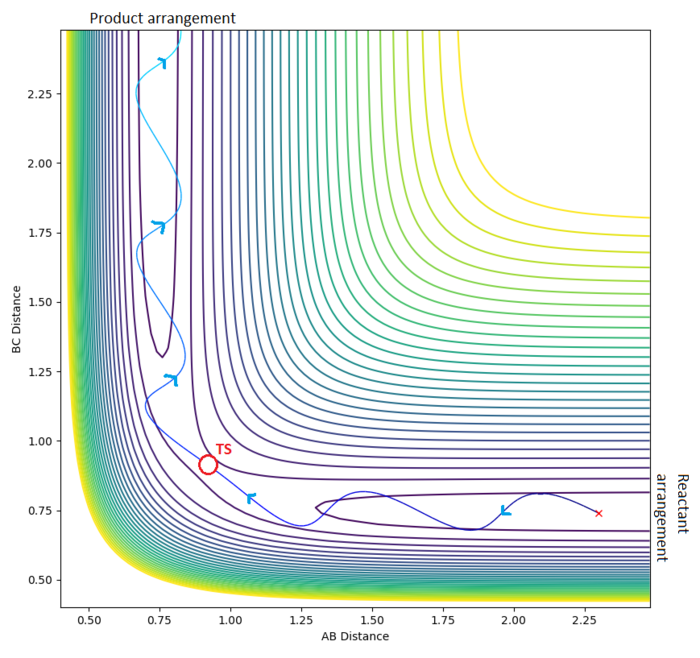
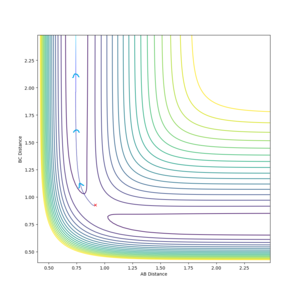
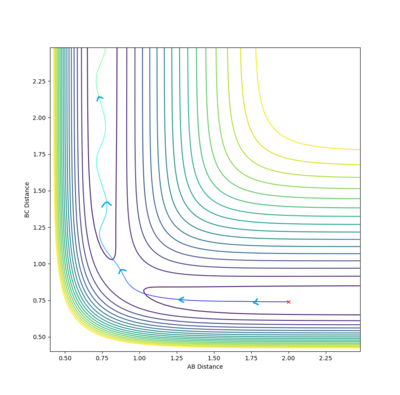
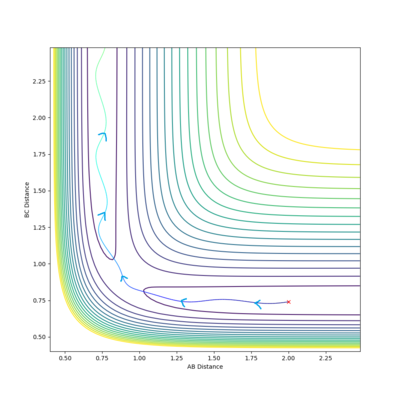
The transition state is located near . To improve upon this value we test a range of values and : akin to seeing which side of the hill a stationary ball rolls down.
| Trial | Internuclear Distances vs Time | Successful? | ||||
|---|---|---|---|---|---|---|
| 1 | 0.9 | 0.9 | 0 | 0 | 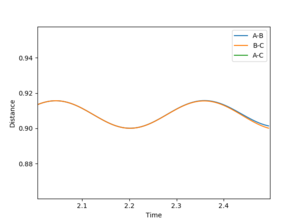 |
✗ |
| 2 | 0.91 | 0.91 | 0 | 0 | 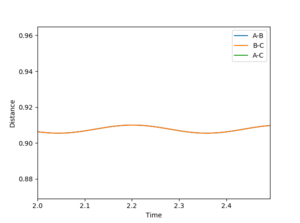 |
✗ |
| 3 | 0.92 | 0.92 | 0 | 0 |  |
✓ |
| 4 | 0.917 | 0.917 | 0 | 0 |  |
✓ |
After 4 tested values (with 500 steps), the transition state occurs when the system has roughly the arrangement: . Specifically, the transition state is a set of configurations (a line on the potential energy surface which passes through a saddle point) associated with a critical geometry such that every trajectory that goes through this geometry reacts.
(This is unfortunately wrong. The transition state is the saddle point. This is one single set of initial conditions at which the system ideally does not move at all. At 0.917, you can still see oscillations as your distances vs time plot shows. At the TS, this plot should give almost perfectly horizontal lines. Fjs113 (talk) 17:51, 14 May 2018 (BST))
(Despite your value for the TS being slightly off, this is very good. Fjs113 (talk) 17:51, 14 May 2018 (BST))
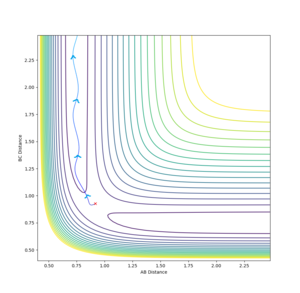
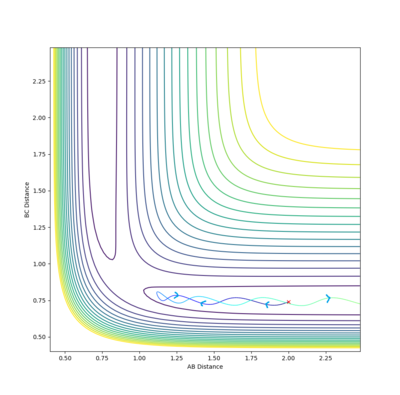
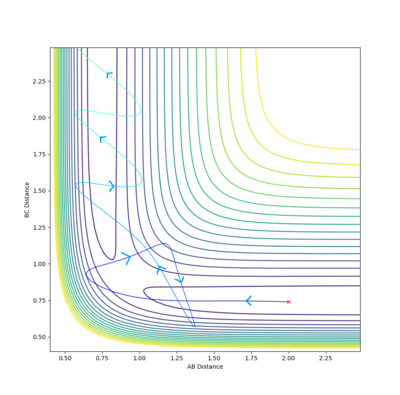
In an attempt to run the minimal kinetic energy reverse reaction, we take the output average radii and momenta from the above calculations. It is expected that the reaction trajectory will almost get to the transition state and then return via the reactant channel. Here is the result of such a collision.
(Careful: Did you mean to say radii? In the classical interpretation used here, we treat atoms as point particles. Fjs113 (talk) 17:51, 14 May 2018 (BST))
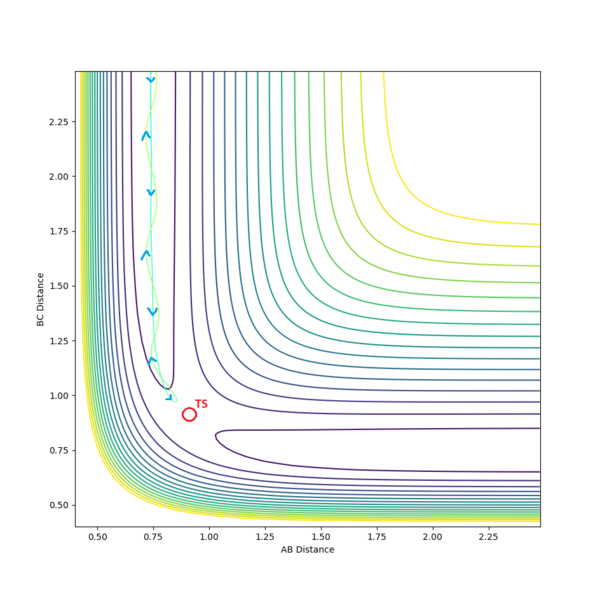
The following table shows the effect of higher collision momenta on the success of a reaction with fixed radii.
The main assumption of transition state theory (TST) is that there is a crucial configuration of no return called the transition state. If molecules pass through this spatial configuration then it is inevitable that they will form products from this encounter.[1] In strict terms, this "inevitability" is only certain once the nascent products are significantly separated from each other.[3]
(This is otherwise known as barrier recrossing, which TST does not take into account. Fjs113 (talk) 17:51, 14 May 2018 (BST))
The experimental result with a calculated energy of -84.96 (see above table) does not agree with TST predictions because this trajectory crosses through the transition state several times but does not afford products.
(Here, we asked you to comment on the reaction rate predicted by TST compared to experiments. You're on the right track, but haven't actually stated that TST will overestimate the reaction rates and why. Fjs113 (talk) 17:51, 14 May 2018 (BST))
F + H2 H + HF
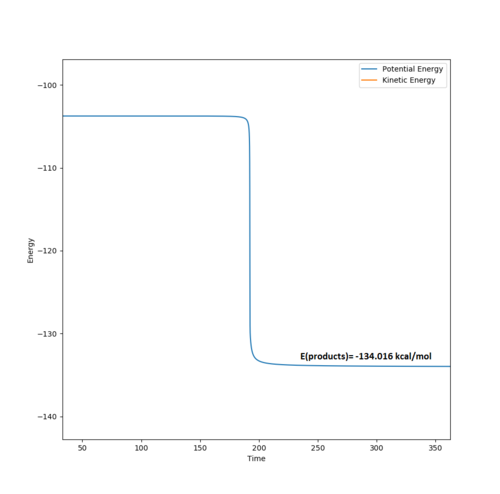
It is known that the F + H2 H + HF reaction is highly exoergic (exothermic) with a value of -32 kcal/mol.[4] The MEP calculated value (see later) of -134.016-(-104.004)= -30.012 kcal/mol for the reaction is accurate and correctly implies that the H-F bond is much stronger than the H-H bond.
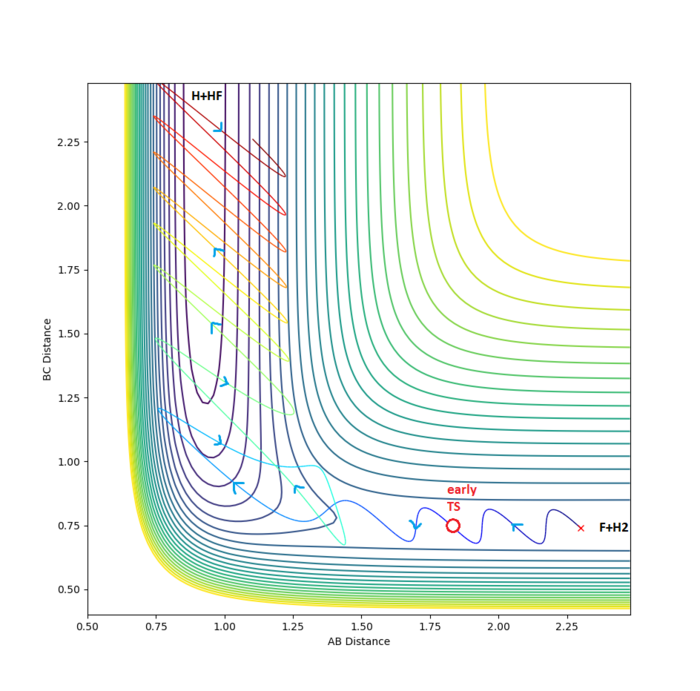
For the successful trajectory above, energy is channelled efficiently into high states of HBFA vibration (shown by the oscillating AB distance). This high vibrational excitation in product HBFA arises from the release of HC-HB bond repulsion while the HB-FA distance is still large; the large zero point vibration in HCHB introduces variability in the observed HBFA vibrational distribution.[5] In simpler terms, trajectories that give rise to large vibrational energy of product(s) typically cut the corner of the energy surface and approach the exit valley from the side.
One way of confirming the formation of vibrationally excited HBFA is the method of infrared chemiluminescence in which emission of infrared radiation can be detected as HBFA returns to its ground state. The populations of the vibrational states of the product can then be determined. An alternative method relies on laser-induced flourescence in which HBFA is excited from a specific vibration-rotation level using lasers. Crossed molecular beams can also be employed.[1]
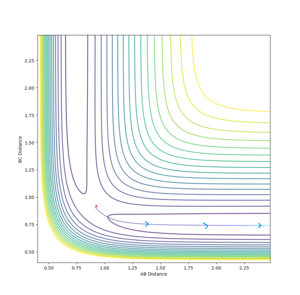
By running 20 Dynamics calculations (1000 steps, , and ), the approximate location of the early transition state for this reaction was determined to be , (see above diagram). The early transition state shows that this reaction surface is attractive and it is well known that such surfaces proceed more efficiently if the energy supplied is largely translational.[1]
The energy of the products was determined using an MEP calculation (400,000 steps, , and ) to give -134.016 kcal/mol (see diagram below).
The energy of the reactants was determined using an MEP calculation (400,000 steps, , and ) to give -104.004 kcal/mol (see diagram below).
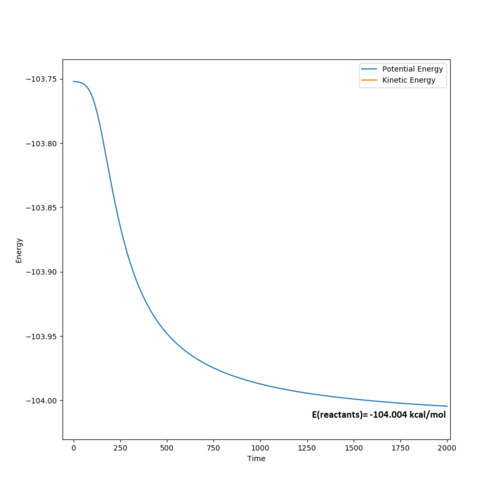
The energy of the transition state, , in both energy level diagrams (above) is -103.752 kcal/mol. This means that the activation energy of the reaction, = +0.252 kcal/mol. This very small activation energy is in accordance with the reaction's vigour.
The table below explores the effect of changing the amount of translational energy provided to the system.
| Contour Diagram (, , ) | Total energy | Reactive? | |
|---|---|---|---|
| -3 | 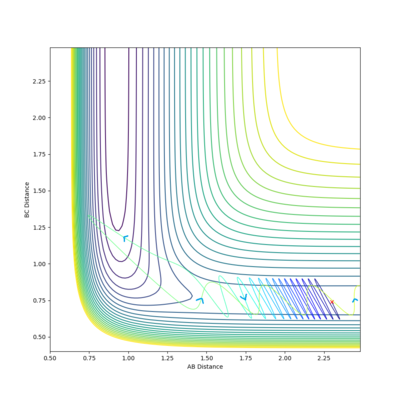 |
-96.254 | ✗ |
| -2 | 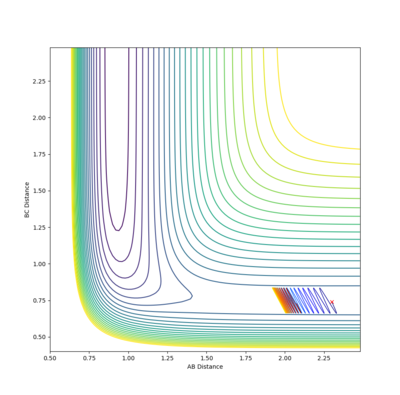 |
-100.754 | ✗ |
| -1 | 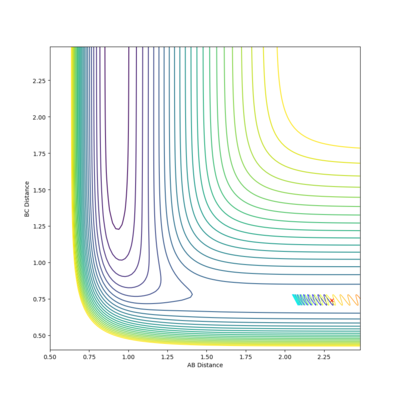 |
-103.254 | ✗ |
| 2 |  |
-98.754 | ✗ |
| 2.3 | 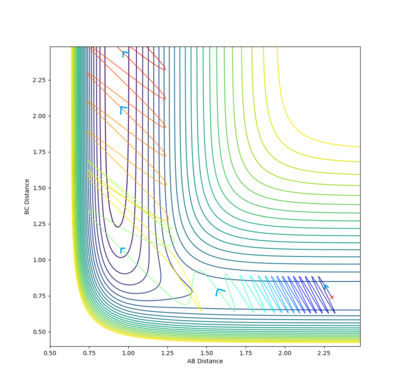 |
-97.314 | ✓ |
| 2.5 | 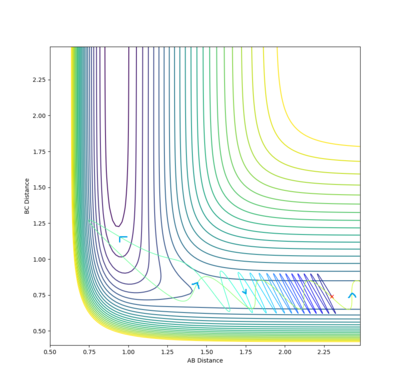 |
-96.254 | ✗ |
| 3 |  |
-93.254 | ✗ |
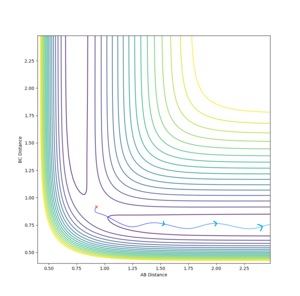
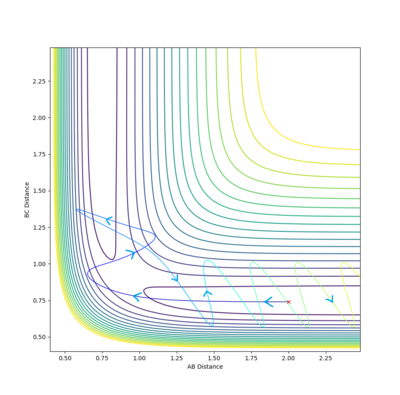
It is clear from the above table that the amount of translational energy provided is not solely responsible for a successful trajectory. Rather, the dominant variable is suggested to be the vibrational phase in HCHB at the moment FA approaches closely.[5] The following contour diagram proves this point.
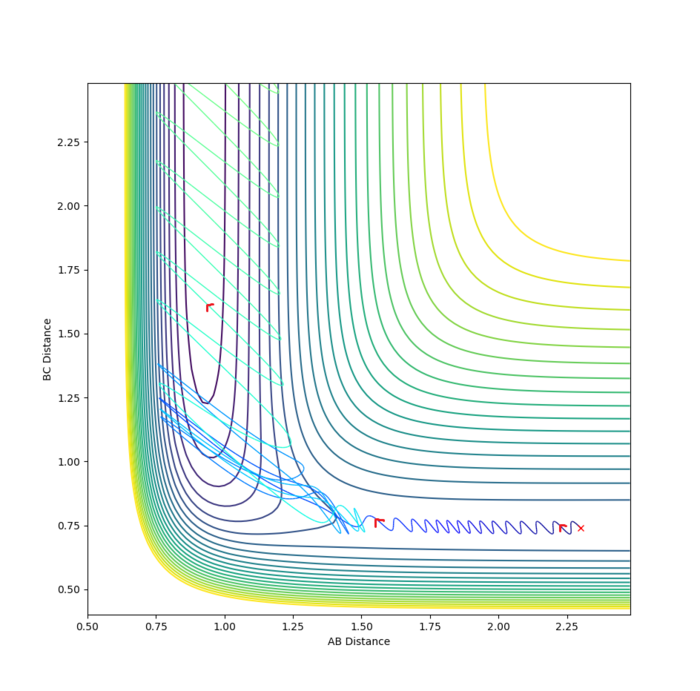
Overall, this reaction of is an example of an attractive surface (with an early transition state) - the success of a trajectory on such a surface, according to Polanyi's rules,[6] is assured by high initial translational energy. However, the results show that other factors such as correct vibrational phase of HCHB are also necessary.
(Hugely impressive! Well done. Fjs113 (talk) 17:51, 14 May 2018 (BST))
H + HF H2 + F
The reverse reaction, H + HF H2 + F, is therefore endoergic with -104.004-(-134.016)= +30.012 kcal/mol.
A successful reaction trajectory across this repulsive (late TS) surface is shown below.
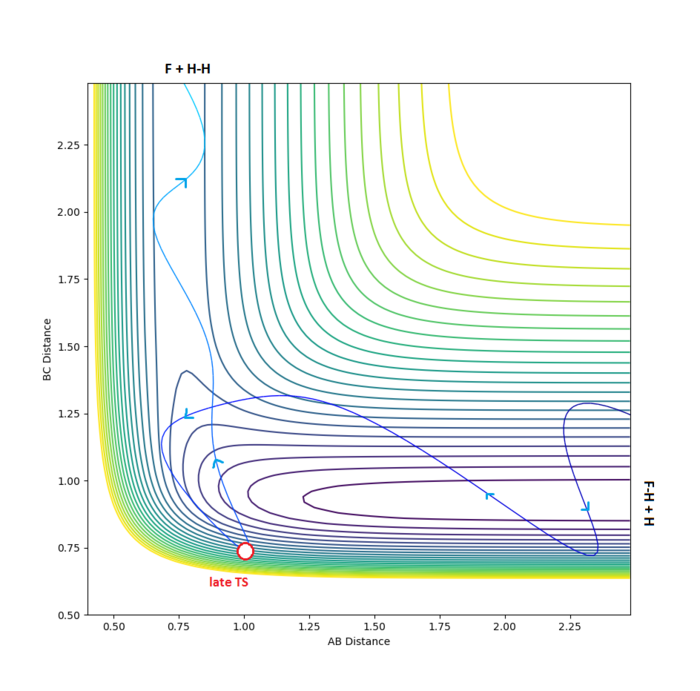
Using similar calculations as used earlier, the approximate location of the late transition state for this reaction was determined to be , (see above diagram).
(How come these values are different from your previous TS? The PES is the same, having just been mirrored along the diagonal, meaning that the TS coordinates are the same, just reversed. Fjs113 (talk) 17:51, 14 May 2018 (BST))
The energy of the reactants was determined using an MEP calculation to give -133.781 kcal/mol (see diagram below).
(Diagram missing? Fjs113 (talk) 17:51, 14 May 2018 (BST))
The energy of the transition state, is -74.1589 kcal/mol. This means that the activation energy of the reaction, = +59.622 kcal/mol.
(The TS energy is the same regardless of forward or backward reaction. Fjs113 (talk) 17:51, 14 May 2018 (BST))
References
- ↑ 1.0 1.1 1.2 1.3 P. Atkins and J. de Paula, Atkins' Physical Chemistry, Oxford University Press, Oxford, 10th edn., 2014, ch. 21, 881-928.
- ↑ Wolfram Mathworld, http://mathworld.wolfram.com/SecondDerivativeTest.html, (accessed May 2018).
- ↑ R. D Levine, Molecular Reaction Dynamics, Cambridge University Press, Cambridge, 2009, 202-212.
- ↑ J. Chen, Z. Sun and D. H. Zhang, J. Chem. Phys., 2015, 142, 1-11.
- ↑ 5.0 5.1 J. C. Polanyi and J. L. Schreiber, Faraday Discuss. Chem. Soc., 1977, 62, 267-290.
- ↑ Z. Zhang, Y. Zhou, D. H. Zhang, G. Czakó and J. M. Bowman, J. Phys. Chem. Lett., 2012, 3, 3416-3419.