Rep:Mod:efr114
Using Gaussian to Determine and Characterise Transition States of Pericyclic Reactions
Using the computational chemistry computer program Gaussian 09W, there are several methods to determine the Transition state. A transition state is the structure with the highest energy in a reaction. It can be modeled in 2D with a simple reaction profile (Figure 2.2) or in 3D where the the reaction proceeds along a minimum energy pathway on 3D potential energy surface. The transition state(s) of a reaction can provide information on the reaction mechanism, thermodynamics, kinetics and a vast amount of other information. Nf710 (talk) 10:54, 21 November 2016 (UTC) Good understanding of the chemistry. You should have defined a TS
Excercise 1: Reaction of Butadiene with Ethylene
| The cycloaddition between butadiene and ethylene is the most simple Diels-Alder reaction. A Diels-Alder reaction is the [4+2] cycloaddition of a diene and a dieneophile. The reaction is reversible, concerted and the forward reaction is exothermic. Due to its concerted nature, the reaction is a single step reaction with one transition state making, the reaction profile simple and easy to analyse.
For all experiments, Method 2 was used to determine the transition state, this was due to repetitive attempts using Method 1 being unsuccessful and enough information was known about the transition state that Method 3 was deemed unnecessary. |
Summary Table
| Reaction stage | Chemical Name | Skeletal Structure | Jmol animation | ||
|---|---|---|---|---|---|
| Reactant | Butadiene | 
|
|||
| Reactant | Ethene | ||||
| Transition State | N/A | 
|
|||
| Products | Cyclohexene | 
|
Table 1.1: A Summary Table of the Structures involved in the reaction
Frequency Calculation
| A DFT/631G opt-freq calculation was performed on the transition state, where one imaginary frequency was produced at -526.7 UNITS which is visulised in Figure 1.1 below. This vibration corresponds to the formation of the products and suggests that the transition state is correct. |
Figure 1.1: A Jmol animation of the imaginary frequency the butadiene/ethene reaction

Reaction IRC
| Intrinsic Reaction Co-ordinate (IRC) calculations are an extremely usaeful tool in Gaussian. An IRC calculation 'begins' at the transition state, the calculation then follows the minimum energy pathway, either forward toward the product/s or backwards towards the reactants.
If an IRC is performed in both directions - a reaction profile can be constructed, seen in figure 1.2, an image which demonstrates the exothermic nature of the forward Diels-Alder reaction due to the difference in energy between reactants and products. |
Molecular Orbitals and their interactions
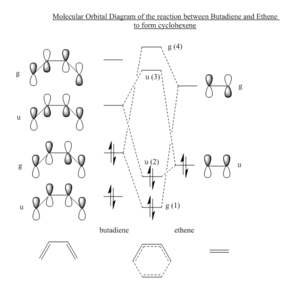
| A molecular orbital diagram of this reaction can be particularly illuminating – the molecular orbitals of both ethane and butadiene can be labelled with the symmetry labels gerade (g) or ungerade (u)-as seen in Figure 1.3. These two molecular orbitals then combine when the two molecules react, however molecular orbitals must have the same symmetry label to combine and only g-g and u-u interactions are allowed.
Two situations could arise from this molecular orbital diagram: the HOMO of the butadiene (g) could react with the LUMO of the ethene (g) or the HOMO of the ethene (u) could react with the LUMO of the butadiene(u). The u-u HOMO-LUMO interaction is less favourable than the g-g HOMO-LUMO interaction as the g-g interaction has a lower energy difference between the two MO’s and is therefore a more favourable interaction. This is confirmed by visualisations of MO's calculated using Gaussian 09W. The transition state LUMO and HOMO (Figure 1.4) appear to be a linear combination of the LUMO of butadiene and the HOMO of ethene, confirming that the u-u interaction is the most favourable for this reaction. As the molecular orbitals of the reactants combine, molecular orbitals of the transition state and product are formed. The HOMO and LUMO of the transition state can be seen in Figure 1.4. |
| LUMO -TS | HOMO -TS | ||||
|---|---|---|---|---|---|
Figure 1.4: Jmol Animation of the HOMO and LUMO of the Transition State of the Reaction
| LUMO Butadiene | HOMO Butadiene | LUMO Ethene | HOMO Ethene | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Figure 1.5: Jmol animations of the HOMO and LUMO of the reactants, butadiene and ethene
Carbon Carbon Bond Lengths through the reaction
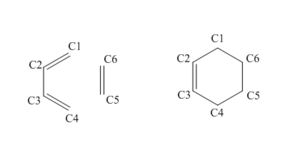
| Bond Involved | Bond Length in Reactants /Å (3 d.p.) | Bond Length in TS /Å (3 d.p.) | Bond Length in Product /Å (3 d.p.) |
|---|---|---|---|
| C1-C2 | 1.335 | 1.383 | 1.500 |
| C2-C3 | 1.468 | 1.407 | 1.338 |
| C3-C4 | 1.335 | 1.383 | 1.500 |
| C4-C5 | N/A | 2.272 | 1.540 |
| C5-C6 | 1.327 | 1.386 | 1.541 |
| C6-C1 | N/A | 2.272 | 1.540 |
Table 1.2: A table of the different carbon carbon bond lengths involved in this reaction.
| Numbering of the carbon atoms can be seen in Figure 1.6 An average bond length for a single carbon-carbon bond is 1.50 Å. An average bond length for a double carbon-carbon bond is 1.33 Å. The Van der Waals radius for carbon is around 1.70 Å
C2-C3 is a single bond in the reactants and a double bond in the product, this is reflected by the change in bond lengths, the bond length changes from 1.468 Å to 1.338 Å meaning the calculations are concordant with theory. C5-C6 starts as a double bond and transforms to a single during the progress of the reaction. C1-C2 and C3-C4 start as double bonds in the reactants and are single bonds in the product. The lengths reflect the concerted, synchronous nature of the Diels-Alder reaction, both bonds stay the same length as each other in the reactants, products and TS.The symmetric relationship between C6-C1 and C4-C5 also demonstrate the synchronous formation of both TS and products. |
Nf710 (talk) 10:57, 21 November 2016 (UTC) This section was done well. Nice formatting ands use of jmols.
Exercise 2: Reaction of Benzoquinone with Cyclopentadiene
| The introduction of benzoquinone makes the Diels-Alder more interesting, there are now two stereoisomers formed as products. The two products are diastereomers- the exo-adduct and the endo-adduct. Technically Benzoquine is a unsymmetrical dienophile, despite being a symmetrical molecule, which gives rise to the exo-endo isomerism. |
| Reaction stage | Chemical Name | Skeletal Formula | Jmol Applet | ||
|---|---|---|---|---|---|
| Reactant | Cyclopentadiene | 
|
|||
| Reactant | Benzoquinone | 
|
|||
| Transition State | N/A | 
|
|||
| Product-Exo | Adduct-Exo | 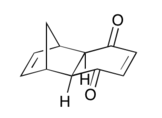
|
|||
| Product-Endo | Adduct-Endo | 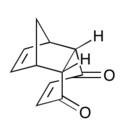
|
Table 2:1A Table displaying the different componants involved in the reaction.
IRC
| Figure 2.1 displays the reaction profiles of the reaction between Benzoquinone with Cyclopentadiene. These profiles were produced using IRC calculations using Gaussian 09W and show that both the exo and the endo cycloaddition reactions are endothermic as the products is significantly higher in energy than the reactants which contrasts the simple butadiene/ethene cycloaddition which was exothermic. |
| Exo | Endo |
|---|---|

|

|
Figure 2.1 Reaction profiles for the endo and exo cyloaddition
Frequency Calculation
| A DFT/631G opt-freq calculation was performed on the transition state, where one imaginary frequency was produced at -815.64 UNITS which is visualized in Figure 2.2 below. This vibration corresponds to the formation of the products and suggests that the transition state is correct. |
| Exo-Front View | Exo-Side View | Endo-Front View | Endo-Side View |
|---|---|---|---|

|

|

|

|
Figure 2:2 Gifs representing the imaginary vibration produced in the Gaussian Calculations NB: Please click on images to view gif of vibrations
Molecular Orbitals
| The reaction of butadiene and ethene in Experiment 1 was the most simple Diels-Alder, there were no other functional groups involved apart from the essential reacting groups. In Experiment 2, the reaction is still a [4+2] cycloaddition, a Diels-ALder reaction, however the reactants of are more complex- altering the reaction pathway.
The carbonyls in benzoquinone are electron withdrawing, resulting in a electron poor dienophile, lowering the energy of the HOMO and LUMO of the dienophile. The ring strain in the cyclopentadiene destabilises the diene and increases the energy of the HOMO and LUMO of the diene. The energy changes in the HOMO's and LUMO's result in the Diene HOMO and Dienophile LUMO energy gap decreasing and the dienophile HOMO and dienophile LUMO increasing. This creates a "normal electron demand" with an electron rich diene and an electron poor dienophile with a small energy gap between the Diene HOMO and the dienophile LUMO resulting in this interaction. The visual of the calculated HOMOs and LUMOs of the transition state give the appearance of a linear combination of the cyclopentadiene HOMO and the benzoquinone LUMO. This observation agrees with theory that the interaction is between the cyclopentadiene HOMO and the benzoquinone LUMO. |
| Exo HOMO | Exo LUMO | Endo HOMO | Endo LUMO | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
(The TS MOs that you have are from minimisation calculations. For any TS you need to specify opt=(TS,calcfc) if you are going to optimise it Tam10 (talk) 15:46, 9 November 2016 (UTC))
Figure 2.3: A visualisation of the HOMO and the LUMO of both the exo and endo transition states
| Cyclopentadiene HOMO | Cyclopentadiene LUMO | Benzoquinone HOMO | BenzoquinoneLUMO | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Figure 2.4: A visualisation of the HOMO and the LUMO of both products
Thermochemistry
Table 2.2 A table displaying the reaction energies of the reactants, TS and products for both the endo and exo reactions. All energies are in the unit of kJ mol-1
| Cyclopentadiene | Benzoquinone | Sum of Reactants | Transition State | Product | Activation Energy | Gibbs Free Energy | |
|---|---|---|---|---|---|---|---|
| Endo reaction | 293.2 | 14.5 | 307.8 | 473.2 | 287.1 | 165.5 | 20.7 |
| Exo reaction | 293.2 | 14.5 | 307.8 | 475.8 | 283.8 | 168.0 | 24.O |
| The thermochemistry of the reaction can be determined by running frequency calculations on the chemical species. The energies were originally given in Hartree per atom and had to be converted to kilojoule per mole. The thermodynamic product is the reaction with the lowest Gibbs Free Energy and the kinetic product is the reaction with the lowest activation energy. The endo product is therefore the kinetic product and the thermodynamic product. In terms of sterics, this should not be the case as there is much more oppurtunity for steric clashes in the endo product than in the exo product, however as the endo product is the most stable, it suggests that secondary orbital interactions are occuring which stabilise the endo product. |
Nf710 (talk) 11:18, 21 November 2016 (UTC) You have come to the correct conclusion with a well weighted argument. But I dont know what you did with your energies but they significantly wrong. however the rest of the exercise was good.
Exercise 3: Diels Alder vs Cheletropic

|
|
Apologies for the lack of Experiment 3, I realised that my TS for the Diels-Alder was completely wrong at the last minute when i was trying to run an IRC. I have done the two other isomers however but no analysis has been done due to frantically trying to sort the last one out.
Conclusion
Gaussian 09W is an extremely useful program for calculating transition states-not only does it allow precise transition states to be calculated without in depth knowledge of computational chemistry, but it also allows analysis of the transition state using different techniques such as IRC, frequency analysis and thermochemistry calculations. Despite its many advantages Gaussian 09W and GaussView are still complex programs to use and even after extensive use can be time-consuming to perform calculations and hence wouldn't be appropriate for "bulk" analysis of a large range of reactions, rather it is more appropriate for in-depth anddetailed analysis.
