Rep:Mod:eb108m3
Module 3- Transition states and Reactivity
Introduction
The Cope Rearrangement
The Cope Rearrangement is an example of a [3,3]-sigmatropic shift rearrangement and is generally accepted to proceed in a concerted manner via either the "chair" or "boat" transition states. This project examines the Cope rearrangement of 1,5-hexadiene to determine the preferred energy minima. This is done by locating the low-energy minima and transition structures on the 1,5-hexadiene potential energy surface. Computational methods are used to calculate the energies of the structures and transition states.
 |
Optimising the Reactants and Products
1,5-hexadiene has many conformers with the central four atoms arranged in either the anti or the gauche conformation. Several of these structures were optimised in GaussView using the HF/3-21G level of theory and were then symmetrized to find the point group.
| Conformation | ||||||
|---|---|---|---|---|---|---|
| Energy/ a.u. | -231.692535 | -231.690971 | -231.689071 | -231.689160 | -23`.691667 | -231.69266 |
| Point Group | Ci | C1 | C2h | C1 | C2 | C1 |
It is expected that the gauche conformers would be higher in energy. This is because the anti conformers have the lowest steric hindrance as the carbons are kept as far from each other as possible. However, the conformer with the lowest energy is in fact a gauche conformer (gauche 3). This is thought to be due to favourable Van der Waals Hydrogen interactions as well as C-H σ* - C=C π orbital overlap.[1].
The Ci anti conformer was optimised again but using a higher level of theory; B3LYP/6-31G* DFT. Parameters of the two structures were compared.
| Property | HF/3-21G | B3LYP/6-31G* |
|---|---|---|
| Point group | Ci | Ci |
| Energy (a.u.) | -231.6925353 | -234.611711 |
| C=C bond length (Å) | 1.32 | 1.33 |
| sp2C(2)-sp3C(3) bond length (Å) | 1.50 | 1.51 |
| C(3)-C(4) bond length (Å) | 1.55 | 1.55 |
| C(1)-C(2)-C(3) angle (o) | 124.8 | 125.3 |
| C(2)-C(3)-C(4) angle (o) | 111.4 | 112.7 |
| C(1)-C(2)-C(3)-C(4) dihedral angle (o) | 114.7 | 118.3 |
The energy was calculated as being more negative for the optimisation using a higher level of theory (B3LYP/6-31G*) indicating that this is the more accurate method. However, the energies cannot be compared because they were calculated using different levels of theory. The largest difference between the two structures was in the dihedral angles of C(1)-C(4) but overall the optimisation methods gave very similar results. The bond lengths compared well to the expected bond lengths (C-C 1.54Å, C=C 1.34Å[2]).
Frequency Calculation
Frequency analysis can be used to determine whether a structure has been correctly optimised. If all the vibrations are positive then the structure is indeed at an energy minima, if there is one negative vibration the structure is in a transition state. A frequency analysis was carried out on the Ci anti conformer that had been optimised using the DFT, B3LYP/6-31G* method and basis set. Table 3 shows some of the key vibrations from the optimisation.
 |
These frequencies were calculated using a harmonic potential approximation instead of an anharmonic potential and so have a 10% error associated with them. A higher level basis set would be needed to improve the accuracy.
The output file from the frequency calculation contains information about the thermodynamic and kinetic parts that make up the energy of the molecule. This allows the enthalpy and free energy of dissociation to be calculated.
| Type of energy | Value (a.u.) |
|---|---|
| Zero-point correction | 0.142493 |
| Thermal correction to energy | 0.149845 |
| Thermal correction to enthalpy | 0.150790 |
| Thermal correction to Gibbs free energy | 0.110895 |
| Sum of electronic and zero-point energies | -234.4691219 |
| Sum of electronic and thermal energies | -234.462867 |
| Sum of electronic and thermal enthalpies | -234.460922 |
| Sum of electronic and thermal freeeEnergies | -234.500817 |
The meaning of the terms in table 4 are as follows:
- Sum of electronic and zero-ponit energies - the potential at 0 K including the zero-point vibrational energy (E=Eelec + ZPE)
- Sum of electronic and thermal energies - the energy at 298.15 K and 1 atm of pressure (standard conditions) which includes the translational, rotational and vibrational energy modes (E= E +Evib +Erot +Etrans).
- Sum of electronic and thermal enthalpies - contains the correction for RT (H=E +RT)
- Sum of electronic and thermal free energies - includes the entropic contribition to free energy (E=H -TS)
Optimising the 'Chair' and 'Boat' transition structures
It is possible to use computational methods to optimise not just the reactants and products but the transition states as well. In the Cope rearrangement the reaction can go through either a "chair" (C2h) or a "boat" (C2v) transition state. An allyl fragment (CH2CHCH2) was drawn out and optimised using the HF/3-21G level of theory. Next, two optimised allyl fragments were placed together to form the chair transition state making sure that the terminal carbons were around 2.2Å apart. This transition state was then optimised using two different methods. The first is the TS(berny) method whereby the force contant matrix (the Hessian) is calculated and then recalculated through optimisation. The initial guess has to be accurate or the resulting structure will be at a false minima. The second method in the frozen coordinate method in which the reaction coordinate is "frozen" whilst the rest of the molecule is optimised. The molecule is then unfrozen and the transition state is optimised again. In this method it is not necessary to calculate the whole Hessian. Both methods used the HF/3-21G level of theory.
The TS(berny) method
An "Opt + Freq" calculation was run with the "optimization to a TS (Berny)" option selected. The force constants were calculated "once" and "Opt=NoEigen" was added into the addition keywords section. The energy of this transition state was calculated to be -231.619322 a.u. and looking at the frequencies showed that there was one negative frequency at 818 cm-1. This vibration corresponded to the Cope Rearrangement. The distance between the terminal carbons was 2.0204Å.
 |
The frozen coordinate method
The distance between the terminal carbons on the allyl fragments was set at 2.2Å and an optimization run to a minimum. Once the molecule had optimised it was then re-optimised except that the "derivative" option was chosen for the terminal allyl carbons and the calculation was run optimised to a TS (Berny) state using the same parameters as before. The energy of the TS was calculated to be -231.6193222 a.u. and there was also a negative frequency at 818 cm-1 corresponding to the Cope Rearrangement. The distance between the terminal carbons was 2.0207Å. compare the two TS
Optimising the "boat" transition state
The TS(QST2) method was used to calculate the transition state for the boat structure. The Ci anti 1,5-hexadiene was used as the reactant and the product molecule but all the atoms were renumbered so that the atoms in the product were in the correct place. This job was submitted but did not finish correctly. This was because these conformations were too far from the correct structure of the transition state to converge.
| Reactant | Product |
|---|---|
 |
 |
To combat this, the geometries of the reactant and product were altered so as to be closer to the transition state. The C(2)-C(3)-C(4)-C(5) dihedral angle was set to 0o and the C(2)-C(3)-C(4) (and the corresponding C(3)-C(4)-C(5)) was set to 100o. The job was then resubmitted and this time the transition state was correctly generated. This transition state had an energy of -231.602802 a.u. and one imaginary vibration at -840 cm-1 indicating that the molecule was at a transition state.
IRC
It is very difficult to predict which conformer will result from a transition state but there is a method in Gaussian called IRC (Intrinsic Reaction Coordinate)that follows the minimum pathway along the potential energy surface to the local minima. At each step a small geometric change is made along the direction of the steepest potential gradient. This method can allow the conformation associated with the transition state to be determined. An IRC calculation was submitted using the chair transition state optimised using the frozen coordinate method with the number of steps set to 50. After 25 steps the calculation converged to give a structure with energy = -213.672038 a.u. However, it also had an imaginary frequency at -817 cm-1 indicating that this was still a transition state.
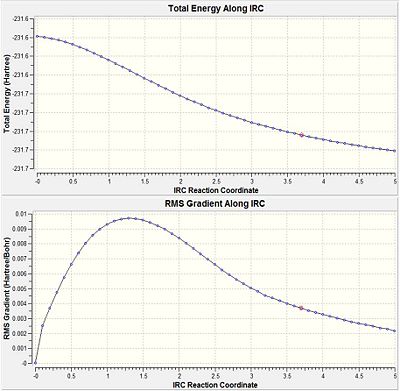 |
To try and find the correct minimum the calculation as rerun as before except that the force constant was computed at each step. This time the calculation converged after 47 steps to give an energy of -231.691649 a.u. All the frequencies were positive indicating a true minimum had been found. This structure corresponded to the gauche C2 conformer.
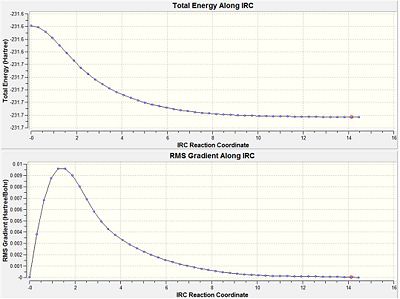 |
Calculating the activation energies
In order to accurately calculate the activation energies of the reaction the transition states were optimised using a higher level of theory; B3LYP/6-31G(d). The log file from each optimisation gave information as to energies at 0K and 295K. When compared to the energies for the starting reactant (anti C2 1,5 hexadiene) the activation energy could be calculated.
| HF/3-21G | B3LYP/6-31G* | |||||
|---|---|---|---|---|---|---|
| Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | |||
| Chair TS | -231.6193 | -231.4667 | -231.4613 | -234.5569 | -234.4149 | -234.4090 |
| Boat TS | -231.6028 | -231.4509 | -231.4453 | -234.5431 | -234.4023 | -234.3951 |
| Reactant (anti2) | -231.693 | -231.540 | -231.533 | -234.612 | -234.469 | -234.462 |
The activation energies were calculated in kcal/mol and are shown in the table below. The energies for each method show a marked difference but cannot be compared.
| HF/3-21G | HF/3-21G | B3LYP/6-31G* | B3LYP/6-31G* | Expt. | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | at 0 K | |
| ΔE (Chair) | 45.81 | 45.18 | 33.89 | 33.26 | 33.5 ± 0.5 |
| ΔE (Boat) | 55.85 | 55.22 | 42.04 | 41.42 | 44.7 ± 2.0 |
compare, which is better method? which TS is lower-prefered
The Diels-Alder Cycloaddition
The Diels-Alder reaction is an example of a [4π+2π] pericyclic cycloaddition between a diene and a dienophile. The HOMO/LUMO on the diene interacts with the HOMO/LUMO on the dienophile. One such Diels-Alder reaction occurs between cis-butadiene and ethylene. First the structures of cis-butadiene and ethylene were optimised using the AM1 semi-empirical molecular orbital method. The HOMO and LUMO MO's of cis-butadiene and ethylene are shown below.
| Reactant | MO | Symmetry |
|---|---|---|
| cis-butadiene HOMO | 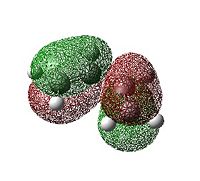 |
asymmetric |
| cis-butadiene LUMO |  |
symmetric |
| ethylene HOMO |  |
symmetric |
| ethylene LUMO |  |
asymmetric |
The transition state in this reaction was optimised using the frozen coordinate method as described above using the Am1 semi-empirical molecualr orbital method. Then it was re-optimised using the higher theory (B3LYP/6-31G(d)).
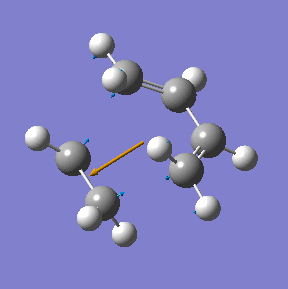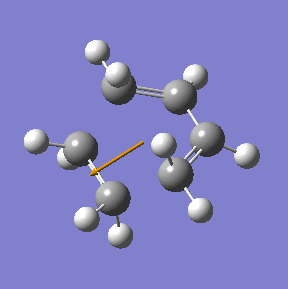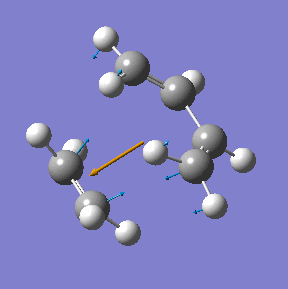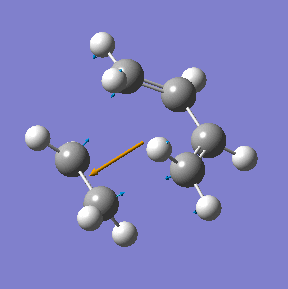
|
| Method | Energy/ a.u. | Imaginary frequency cm-1 | C-C bond length/ Å |
|---|---|---|---|
| AM1 semi-empirical | 0.1162913 | -955 | 2.12 |
| DFT/B3LYP/6-31G(d) | -234.54896 | -524 | 2.27 |
lower: C=C bond length = 1.38, C-C bond length =1/39 higher:C=C bond length = 1.38, C-C bond length=1.40 literature values
The molecular orbitals for the HOMO and LUMO were ...
| Molecular Orbital | Energy (a.u.) | Symmetry | |
|---|---|---|---|
| LUMO | 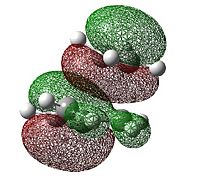 |
0.023 | Symmetric |
| HOMO |  |
-0.323 | Anti-symmetric |
maleic anhydride diels-alder:
lower,endo: energy= 0.00749388 imaginery frequency= -826 higher, endo: energy=
lower, exo:energy= higher, exo:energy=
- ↑ B.G. Rocque, J.M. Gonzales, H.F. Schaefer III, Mol. Phys., 2002, 100, 441[1]
- ↑ http://www.wiredchemist.com/chemistry/data/bond_energies_lengths.html