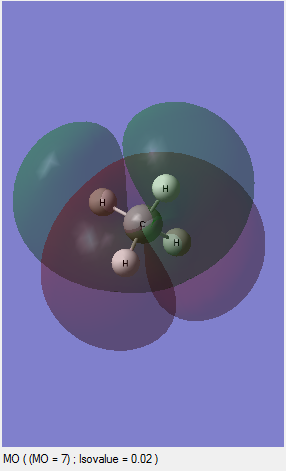Rep:Mod:dy815
NH3 molecule
Molecule name:ammonia
Calculation method: RB3YLYP
Basis set:6-31G(d.p)
Final energy E:-56.55776873 a.u.
RMS gradient norm:0.00000485 a.u.
The point group: C3V
Bond length(N-H):1.01798Å
Bond length(H-H):1.62322Å
Bond angle:105.741degrees
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 |
The optimisation file is liked to here

| mode | frequency |
|---|---|
| Mode1 | 1089.54 |
| Mode2 | 1693.95 |
| Mode3 | 1693.95 |
| Mode4 | 3461.29 |
| Mode5 | 3589.82 |
| Mode6 | 3589.82 |
NH3 molecule vibration
How many modes do you expect from the 3N-6 rule?
6 types.
Which modes are degenerate (ie have the same energy)?
Mode 2 and 3 and mode 5 and 6.
Which modes are "bending" vibrations and which are "bond stretch" vibrations?
Mode 1,2,3 are 'bending' and mode 4,5,6 are 'bong stretch'.
Which mode is highly symmetric?
Mode 4.
One mode is known as the "umbrella" mode, which one is this?
Mode 1.
How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
2.
NH3 molecule charge distribution
The H atom contains 0.373 and the N has -1.125. Since N is more electronegative than H, nitrogen has negative charge but hydrogen takes positive charge.
N2 molecule Analysis
Molecule name:nitrogen
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
E(RB3LYP) :-109.52412868 a.u.
RMS Gradient Norm : 0.00000365 a.u.
Bond length:1.10550Å
Bond angle:180degrees
point group:D*H
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000002 0.001800 YES RMS Displacement 0.000003 0.001200 YES
N2 |
The optimisation file is liked to here
There just one vibration for N2 molecule.
H2 molecule Analysis
Molecule name:hydrogen
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Bond length:0.74309Å
Bond angle:180degrees
RMS Gradient Norm : 0.00012170 a.u.
E(RB3LYP) : -1.17853930 a.u.
point group:D*H
Item Value Threshold Converged? Maximum Force 0.000211 0.000450 YES RMS Force 0.000211 0.000300 YES Maximum Displacement 0.000278 0.001800 YES RMS Displacement 0.000393 0.001200 YES
H2 |
The optimisation file is liked to here
There just one vibration for H2 molecule.
energy for the reaction of N2 + 3H2 -> 2NH3
E(NH3)=-56.55776873 a.u.
2*E(NH3)=-113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)=-1.17853930 a.u.
3*E(H2)= -3.5356179a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.05579088 a.u.= -146.47895544 kj/mol
The product is more stable in this reaction because this reaction is exothermic process which means the energy level of the final product is lower than the starting materials.
CH4 MOLOECULE
Molecule name:Methane
Bond length(H-H):1.09194Å
Bond angle:109.471degrees
Calculation method:RB3LYP
Basis set:6-31G(d,p)
Energy:-40.52401405 a.u.
RMS gradient norm:0.00002267
Point group:TD
Item Value Threshold Converged? Maximum Force 0.000044 0.000450 YES RMS Force 0.000023 0.000300 YES Maximum Displacement 0.000124 0.001800 YES RMS Displacement 0.000066 0.001200 YES
CH4 |
The optimisation file is liked to here
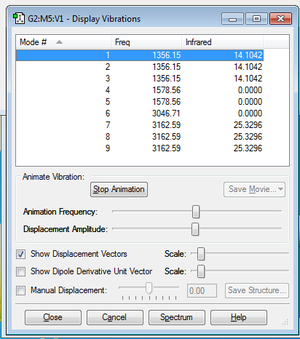
It is shown that there is 9 types of vibration.And there are 3 types of degenerated vibrations, which are mode 1,2,3 and mode 4,5 and mode 7,8,9. The mode1,2,3,4,5 are 'bending' vibration, while the mode6,7,8,9 are 'bond stretch' vibration. The most highly symmetric vibration of the methane molecule is mode6 which you can find the symmetric centre does not change via vibration. Two main peaks are shown in the IR spectrum of methane molecule, which distributes nearly 1350cm-1 and another locates near 3150cm-1.
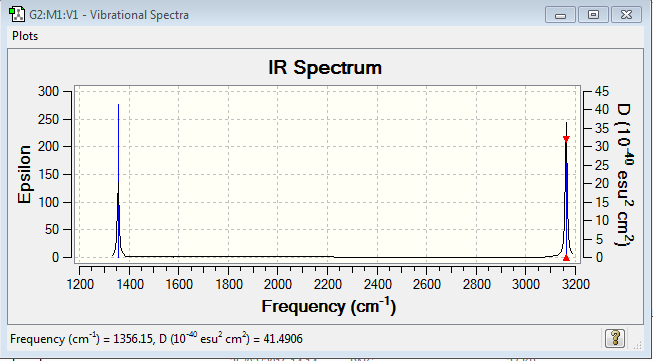 This is the IR spectrum of CH4 molecule.
This is the IR spectrum of CH4 molecule.
| mode | frequency |
|---|---|
| Mode1 | 1356.15 |
| Mode2 | 1356.15 |
| Mode3 | 1356.15 |
| Mode4 | 1578.56 |
| Mode5 | 1578.56 |
| Mode6 | 3046.71 |
| Mode7 | 3162.59 |
| Mode8 | 3162.59 |
| Mode9 | 3162.59 |
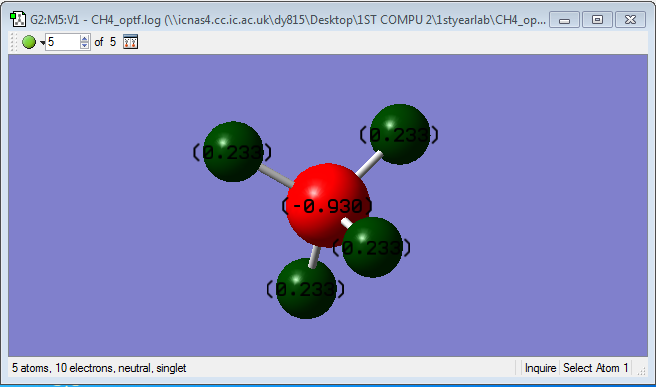
charge distribution of methane
Because carbon atom has more electronegativity, thereby showing in the diagram that the carbon atom carries 0.930 unit of negative charge. While the hydrogen takes 0.230 unit of positive charge.
CH4 Molecular Orbitals
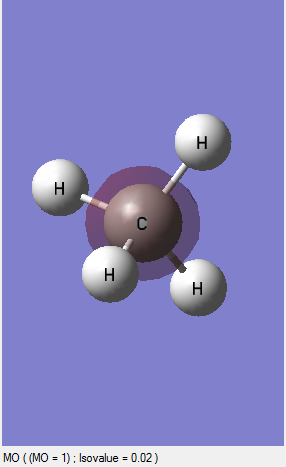
MO1:This is an occupied orbital and this molecular orbital is contributed by the 1S orbital of the carbon. And this molecular orbital is the deepest in energy because the 1S AO of carbon does not involve in the bond formation. In other words, the AO is remained unchanged and located in the most inner of the molecule under the most circumstances(cannot say it located because electrons are moving all the times).
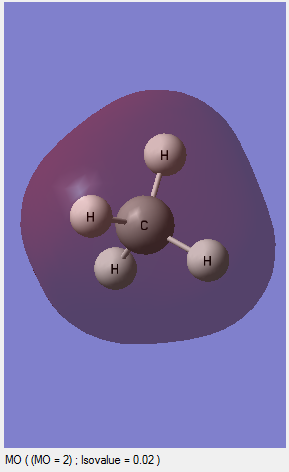
MO2:This is an occupied orbital and this is contributed by the 2S AO of the carbon and 1S AO of the hydrogen. This molecular orbital is given the expression of one C-H bonds which involves 1S of the hydrogen and 2S of the carbon combination.
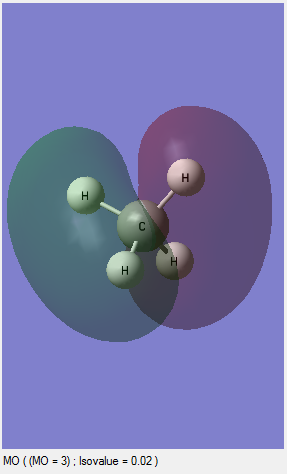
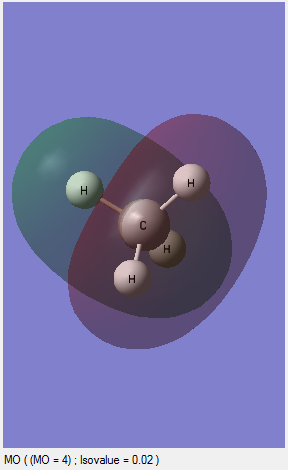
MO3: This is an occupied orbital and this is contributed by one of the 2P orbitals of the carbon and 1S of one of the hydrogens. This molecular orbital is the degenarated form of other two MOs. In other words, there are two other MOs which are also combined by one 2P orbital of the carbon and 1S of one hydrogen. This MO is the HOMO in the molecule because it is the occupied orbital with the highest energy level among those all of occupied orbitals. This orbital represents one of the C-H bonds. The diagrams of other two degenerated MOs are shown above.
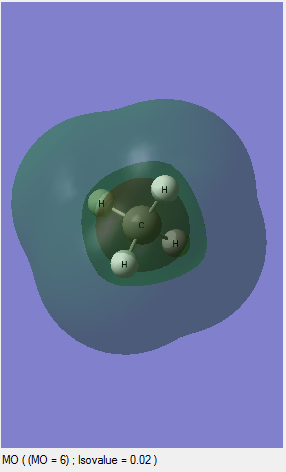
MO6: This is an unoccupied orbital and it is the LUMO for CH4 molecule. This is the antibongding orbital of the MO2 and the MO is formed by 1S of one hydrogen and 2S of the carbon.
MO7: This is an unoccupied orbital and this antibonding orbital is formed by 1s of one hydrogen and 2P of the carbon.