Rep:Mod:ds5517
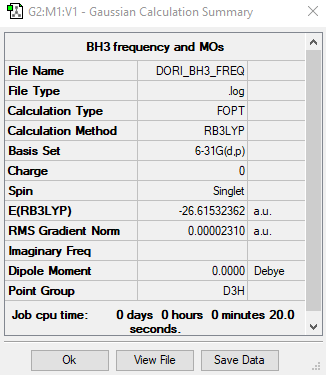
BH3
RB3LYP / 6-31G(d,p)
summary table
item table
Item Value Threshold Converged?
Maximum Force 0.000203 0.000450 YES
RMS Force 0.000098 0.000300 YES
Maximum Displacement 0.000736 0.001800 YES
RMS Displacement 0.000395 0.001200 YES
log file
low frequencies
Low frequencies --- -0.4072 -0.1962 -0.0054 25.2514 27.2430 27.2460 Low frequencies --- 1163.1897 1213.3128 1213.3155
jmol
test molecule |
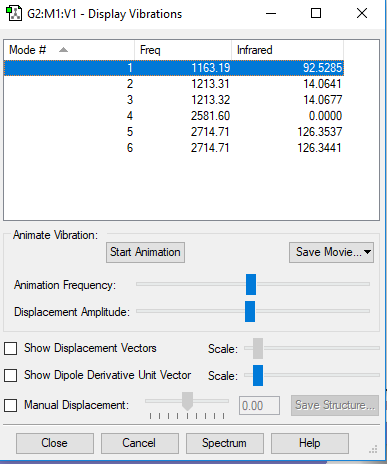
Display vibrations
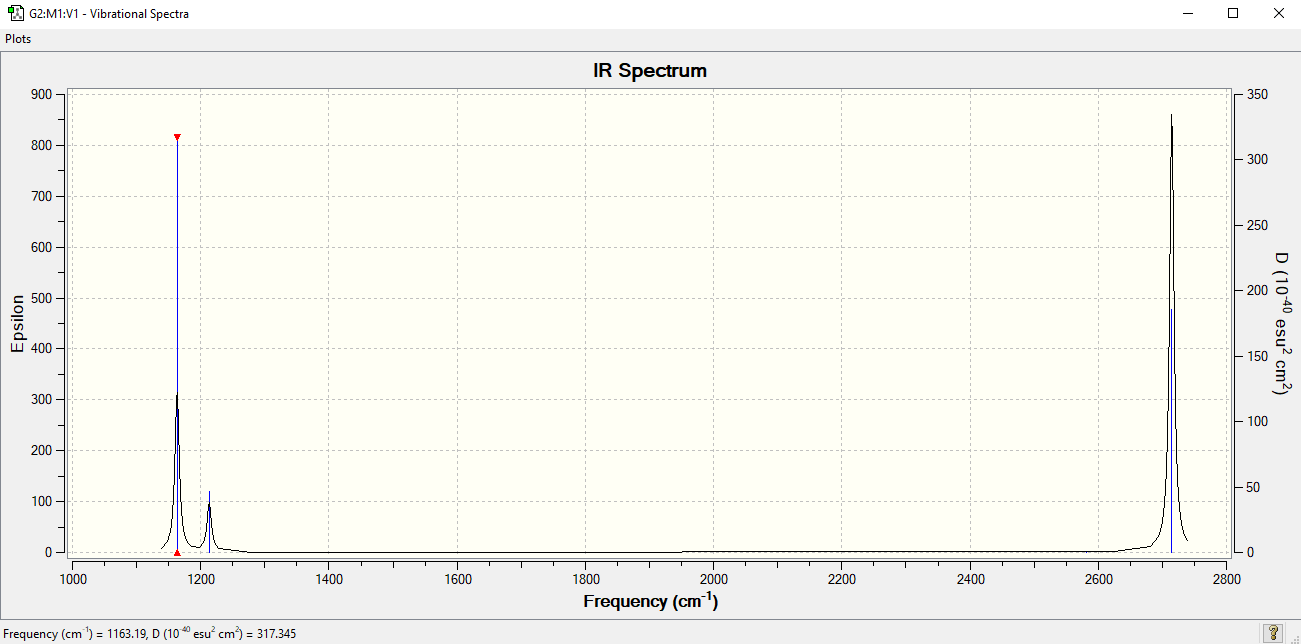
IR spectrum
| Wavenumbers (cm-1) | Frequency | IR active? | Symmetry | Type |
|---|---|---|---|---|
| 1163 | 92 | Yes | A2 | Out-of-plane bend |
| 1213 | 14 | Slightly | E' | Bend |
| 1213 | 14 | Slightly | E' | Bend |
| 2581 | 0 | No | A1' | Symmetric stretch |
| 2714 | 126 | Yes | E' | Asymmetric stretch |
| 2714 | 126 | Yes | E' | Asymmetric stretch |
There are 6 vibrations shown on the table above, however only 3 peaks on the IR spectrum. This is due to the fact that the vibrations at 1213 and 2714 cm-1 are degenerate (resulting in one peak each) and the vibration at 2581 cm-1 is symmetric, thus there is no change in dipole moment and it is not IR visible.
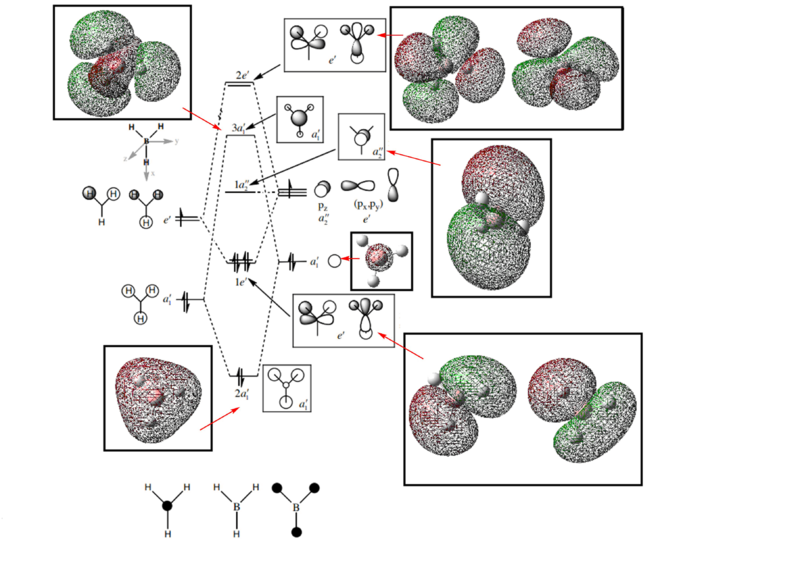
BH3 MO Diagram [1]
Are there any significant differences between the real and LCAO MOs? What does this say about the accuracy and usefulness of qualitative MO theory?
There are no significant difference between the real and LCAO MOs. This indicates that the qualitative MO theory is really accurate and useful in this case, however for less covalent molecules, the accuracy might change.
You've correctly included the calculated MOs on to the diagram which is good. However, the core orbital has been used to incorrectly annotate the a1' AO and it shouldn't have been included on the diagram - if you looked at the energy level of the core orbital then you can see that it is so low that it wouldn't appear on the diagram and is not relevant. To improve it would have also been good to consider some of the differences which do actually occur between the real and calculated MOs. Smf115 (talk) 06:34, 30 May 2019 (BST)
NH3
RB3LYP / 6-31G(d,p)
summary table
item table
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000012 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
Predicted change in Energy=-9.844527D-11
Optimization completed.
-- Stationary point found.
log file
low frequencies
Low frequencies --- -8.5646 -8.5588 -0.0041 0.0455 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
jmol
test molecule |
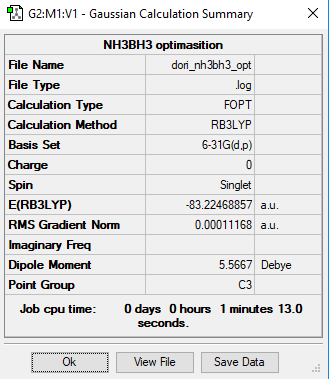
NH3BH3
RB3LYP / 6-31G(d,p)
summary table
item table
Item Value Threshold Converged? Maximum Force 0.000233 0.000450 YES RMS Force 0.000083 0.000300 YES Maximum Displacement 0.000981 0.001800 YES RMS Displacement 0.000370 0.001200 YES Predicted change in Energy=-4.047521D-07 Optimization completed.
log file
low frequencies
Low frequencies --- -0.1775 -0.0266 -0.0049 12.0724 12.1731 37.7614 Low frequencies --- 264.5446 634.5253 639.1516
jmol
test molecule |
Association Energy
E(BH3)= -26.6153 a.u. E(NH3)= -56.5578 a.u. E(NH3BH3)= -83.2247 a.u.
ΔE=E(NH3BH3)-[(NH3)+(BH3)] ΔE= -0.0516 a.u. = -135 kJ/mol
Based on your energy calculation is the B-N dative bond weak, medium or strong? What comparison have you made to come to this conclusion?
Negative value of the association energy means that the formation of the B-N dative bond is favourable. The bond strength is relatively weak compared to C-C single bond (346 kJ/mol), N-N single bond (167 kJ/mol) and to B-B bond (293 kJ/mol). [2]
Correct calculation, good consideration given to the accuracy of the final reported energy and well-referenced comparisons! The supporting calculations are good, just note that the summary tables should have been from the frequency jobs and not the optimisations. Smf115 (talk) 06:41, 30 May 2019 (BST)
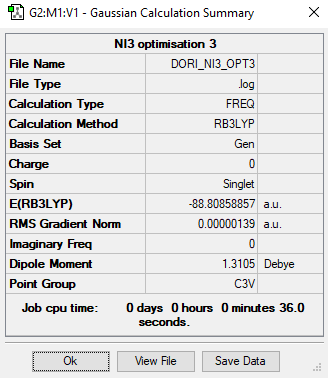
NI3
RB3LYP / 6-31G(d,p)
summary table
item table
Item Value Threshold Converged? Maximum Force 0.000002 0.000450 YES RMS Force 0.000002 0.000300 YES Maximum Displacement 0.000024 0.001800 YES RMS Displacement 0.000014 0.001200 YES Predicted change in Energy=-8.532932D-11 Optimization completed.
log file
low frequencies
Low frequencies --- -12.5520 -12.5458 -6.0044 -0.0039 0.0191 0.0664 Low frequencies --- 100.9969 100.9976 147.3377
jmol
test molecule |
N-I bond length: 2.18 Å
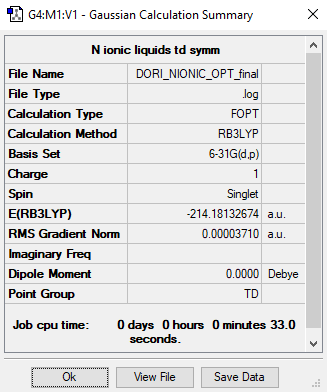
[N(CH3)4]+
RB3LYP / 6-31G(d,p)
summary table
item table
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000039 0.000300 YES Maximum Displacement 0.000922 0.001800 YES RMS Displacement 0.000357 0.001200 YES Predicted change in Energy=-3.033030D-07 Optimization completed.
log file
File:DORI NIONIC FREQMO FINAL.LOG
low frequencies
Low frequencies --- 0.0011 0.0013 0.0014 34.8971 34.8971 34.8971 Low frequencies --- 216.8553 316.1296 316.1296
jmol
test molecule |
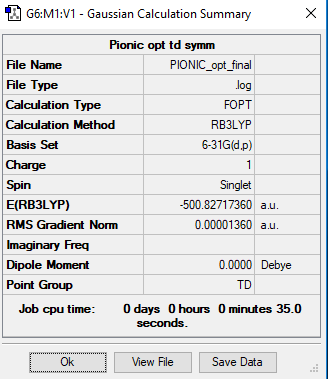
[P(CH3)4]+
RB3LYP / 6-31G(d,p)
summary table
item table
Item Value Threshold Converged? Maximum Force 0.000029 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000110 0.001800 YES RMS Displacement 0.000031 0.001200 YES Predicted change in Energy=-8.481699D-09 Optimization completed.
log file
low frequencies
Low frequencies --- -0.0022 -0.0020 -0.0008 50.7702 50.7702 50.7702 Low frequencies --- 188.1765 213.2027 213.2027
jmol
test molecule |
Charge Distributions
[N(CH3)4]+
| Atom | Charge | Pauling Electronegativity[3] |
|---|---|---|
| Nitrogen | -0.295 | 3.07 |
| Carbon | -0.483 | 2.50 |
| Hydrogen | 0.269 | 2.1 |
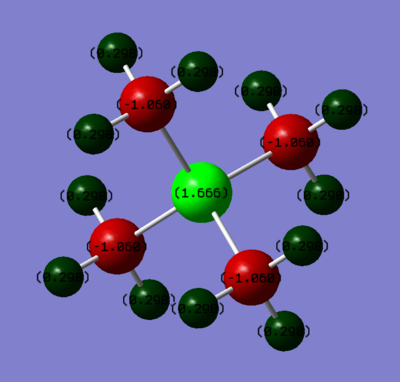
[P(CH3)4]+
| Atom | Charge | Pauling Electronegativity[3] |
|---|---|---|
| Phosphorous | 1.667 | 2.06 |
| Carbon | -1.060 | 2.50 |
| Hydrogen | 0.298 | 2.1 |
Electronegativity describes the ability of the atom to attract electrons. The two tables above show that the electronegativities vary in the following order: N > C > H > P. The largest colour range was used to visualize the charge distributions on the two ions. Hydrogens have the same colour, since the difference in electronegativities between C and H does not change. However, because in [N(CH3)4]+ C binds to the more electronegative N and in [P(CH3)4]+ it binds to an electropositive P atom, C atoms have different colours.
As a result of symmetry (all the hydrogens are related by symmetry, just like the carbons), the charge distribution within a molecule does not change when we go from one H (or C) to another. The charge is equally distributed.
Very good presentation of the NBO charges with referenced electronegativities for each atom. Clear analysis of the charges with regards to the relative electronegativities and to the symmetry! To improve, you could have considered further effects such as why the P and H have such different charges but comparable electronegativities and using the charge values instead of comparing the colour would have been better. Smf115 (talk) 14:00, 30 May 2019 (BST)
What does the "formal" positive charge on the N represent in the traditional picture? On what atoms is the positive charge actually located for this cation?
The "formal" positive charge on the N in the traditional represents the fact that the N has donated its lone pair to form a dative bond with the carbon, therefore became positively charged. However, the calculations show that the positive charge is not located on the nitrogen, but is distributed on the molecule and mostly located on the hydrogens.
Correct observation about where the positive charge actually lies, however, you needed to consider formal electron counting when discussing how the +1 N charge actually arises in the traditional picture. Smf115 (talk) 14:14, 30 May 2019 (BST)
MO visualisation
Good attempt at constructing the FOs and resulting LCAOs and clearly presented. However, the FO and LCAO is incorrect for MO 10 and it might have helped to consider the BH3 MO diagram. You've labelled the main interactions but it would have been good to see an attempt at evaluating the strengths of the interactions and then the overall character of the MO. Smf115 (talk) 14:24, 30 May 2019 (BST)
A very good report with a well presented second section. Smf115 (talk) 14:24, 30 May 2019 (BST)
References
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedMO - ↑ http://www.wiredchemist.com/chemistry/data/bond_energies_lengths.html.
- ↑ 3.0 3.1 Little, E. J., & Jones, M. M. (1960). A complete table of electronegativities. Journal of Chemical Education, 37(5), 231. https://pubs.acs.org/doi/pdf/10.1021/ed037p231