Rep:Mod:dr1615TS
Introduction
In this report three reactions were analysed by gaussview to gather information on the transition states e.g. MOs, reaction barrier and energy and which, if applicable, was the more stable transition state (TS) and product.
The potential energy surface (PES) computed by the software is the basis for these calculations. Within PES the minimum and the transition state can be found. The minimum is where the molecule is optimized to point where all (in the 3N-6 dimensions the PES was computed where N = number of atoms) second derivatives have a positive value when the first derivative is 0. Whereas in the TS the first derivative is still zero but one of the second derivatives is neagative and all other positive due to there being a local maximum as well as the other minimums.
A frequency calculation would show if the optimized structure was at a minimum by showing no negative frequency because there was no negative curvature for that point whereas the TS will have one because there is one negative curvature. If there are more than 1 negative frequencies then the structure optimised was not at a minimum or a TS but just another local maxima/minima in the PES.
The calculations were run either at PM6 or B3LYP or both depending on the complexity of the structure being analysed. All exercises were (initially) run at PM6 as it is a much faster albeit slightly inaccurate method. PM6 is used for the complex structures where an initial optimisation will bring it close to the minimum but then optimizing with B3LYP will be run on it to produce a more accurate optimization.
Nf710 (talk) 10:29, 18 December 2017 (UTC) This is good and correct. But very brief. Some more detail into the quantum methods would have been nice and maybe a picture showing a saddle point etc.
Exercise 1: Reaction of Butadiene with Ethylene
Reaction overview
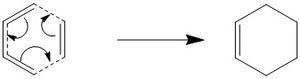
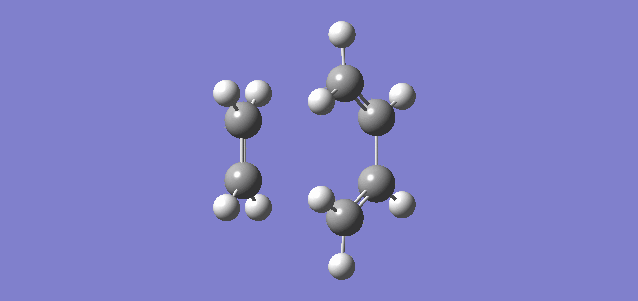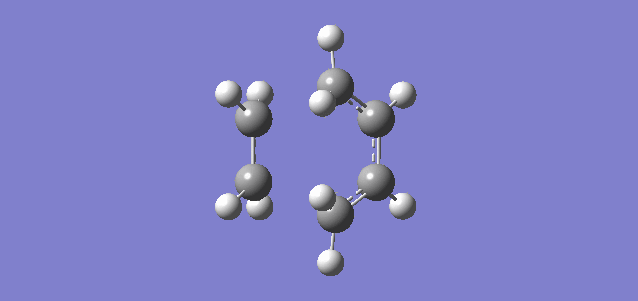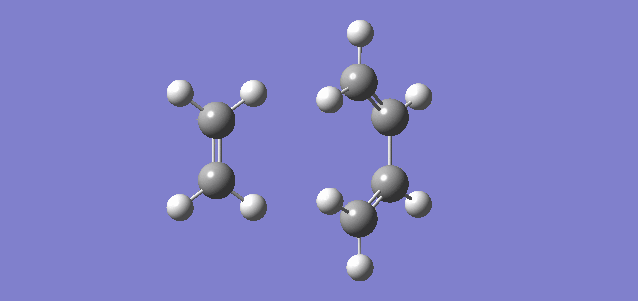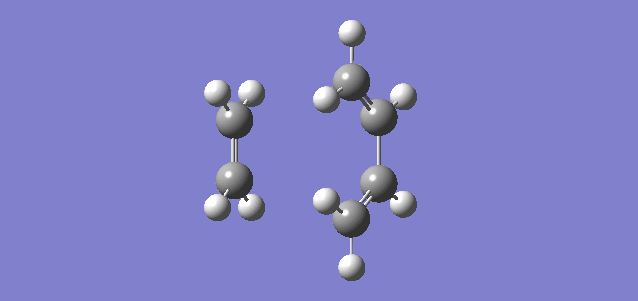
The reaction being carried out in this first exercise is the [4+2] cycloaddition of cis-1,3-butadiene with ethene to form cyclohexene. This reaction is part of a subset of cycloaddition reactions known as the Diels-Alder reaction. the mechanism for this reaction is seen in the image to the left. The transition state of this reaction is analysed to look at the MOs, the change in bond length over the reaction and the bond forming vibration.
Below, the reaction pathway and the bond forming vibration in the transition state can be seen as gifs. The bond forming vibration is the imaginary frequency in the transition state which comes to -948.56 cm-1. It is clear from this that bond forming of both bonds is synchronous.
| Bond forming vibration | IRC |
|---|---|
 |
|
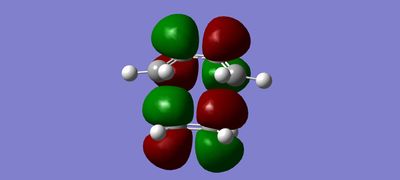
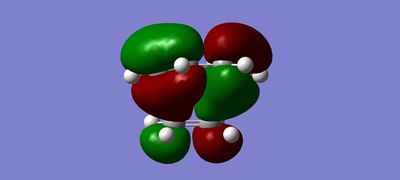
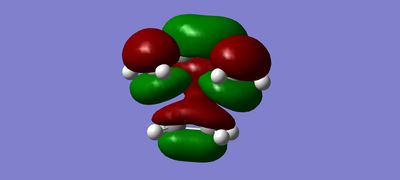
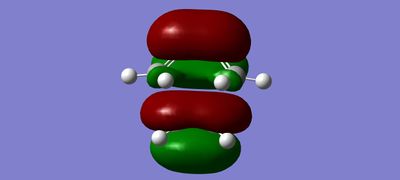
MOs
(Fv611 (talk) The combinations of your fragment MOs are correct, but you have not looked into their relative energies at all. This is a TS MO diagram, so the bonding TS MOs will have a higher energy than both the HOMO of the reactants.)
The MO diagram drawn in chemdraw shows that the symmetric HOMO of the ethene is interacting with the symmetric LUMO of butadiene. In a similar way, the antisymmetric LUMO of ethene is interacting with the antisymmetric HOMO of butadiene. This interaction is known from MO theory that only MOs with same symmetry will interact together. This leads to the formation of 4 molecular orbitals in the transition state, 2 with pairs of electrons and 2 without. This is seen in the MO pictures seen below taken from the computations carried out on the reactants and the transition structure. For a symmetric-antisymmetric interaction the overlap energy is zero because one part of the function will overlap in phase and the other part out of phase meaning overall there is no interaction because the in phase and out of phase cancel each other out. A s/s or a/a interaction have non-zero overlap energy, clearly seen in the calculated MOs, because the whole function will overlap in phase or out of phase depending on the nature of the MOs interacting.

Bond Lengths
(Fv611 (talk) When reporting measurements, you should make clear which value corresponds to what. You should have included a labelled reaction scheme, to be able to identify the bonds corresponding to each reported bond length.)
The carbon-carbon bond lengths in the optimized Butadiene are 1.335 (double bond), 1.468 (single bond), 1.335 Å (double bond). In the optimized Ethene it is 1.327 Å (double bond).
The carbon-carbon bond lengths in the optimized Cyclohexene are 1.337 (double bond), 1.501 (single bond next to double bond), 1.537 (single bond), 1.535 (single bond), 1.537 (single bond), 1.501 (single bond next to double bond) Å. The bond lengths are given by going around the molecule from the double bond.
The carbon-carbon bond lengths in the optimized transition state are 1.380, 1.411, 1.380, 2.115, 1.382, 2.115 Å. 1.380 Å bond lengths are the double bonds in the butadiene fragment going to single bonds, 1.411 Å bond length is the single bond going to double bond in the butadiene fragment, 2.115 Å bond lengths are the new bond being formed between the butadiene and ethene fragments, 1.382 Å is the double bond in ethene fragment turning to a single bond.
As the reaction proceeds from the reactants to the products via the transition state the double bonds present in the reactants increase in length, the single bond decreases in length and a bond forms between the reactants by the distance between them decreasing to the length of a single bond.
The normal length of single bond is 1.54 Å and double bond is 1.34 Å. We can see from this that the bond length for the single bond in butadiene is a bit off, this is due to the conjugation that occurs in the molecule giving that bond a slight double bond character. The double bonds and the single bonds in the product all come come very close to the normal value. The van der Waal's radius of carbon is 1.7 Å, from this we can see that in the transition state that there is some bonding character between all carbons as all the bond lengths quoted in the TS is below the 3.4 Å threshold (2x1.7).
Files
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
Reaction Overview
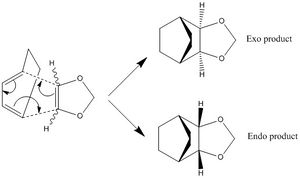
The reaction of these molecule can proceed via two routes labelled Endo-Diels-Alder and Exo-Diels-Alder which have different approaches, transition states and slightly different products. This leads to different reaction barriers and energies which will be analysed below. The interacting MOs in the transition state are also analysed to look at which MOs are occupied and unoccupied. The reaction pathways are shown below to illustrate how the different products are formed. The endo pathway had to be reversed online to show it going from reactants to products rather than what the IRC showed hence the double take at the start of animation. The optimisation of the reactants products and transition state was carried at PM6 first then B3LYP to get accurate representations of the MOs, energies etc. The gifs below are from IRCs run at the PM6 rather than B3LYP because B3LYP takes much longer to converge.
| Exo product formation | Endo product formation |
|---|---|
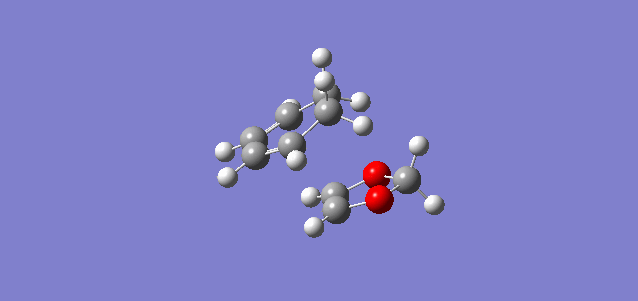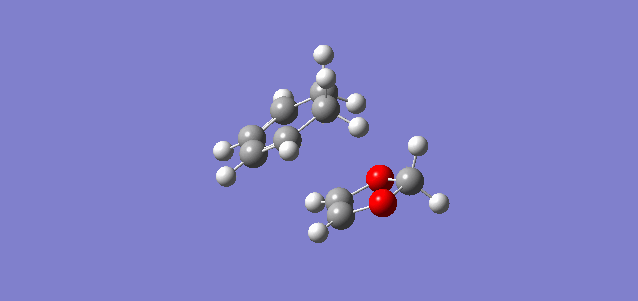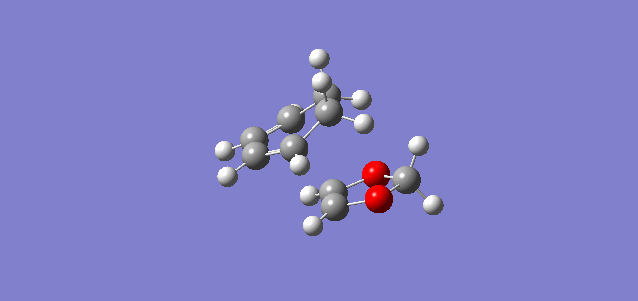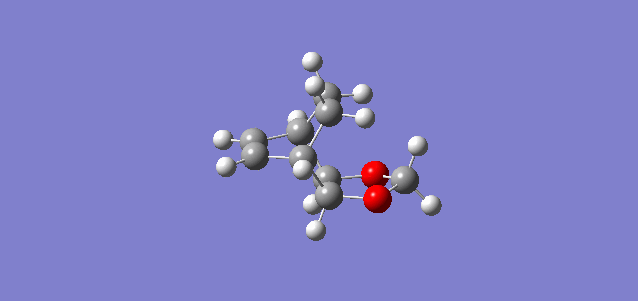 |
|
MOs
(Fv611 (talk) Nice MO diagram, but you should have included a discussion of the difference between the endo and exo case.)
The MOs shown below shows that the HOMO and LUMO of the transition state comes from the combination of symmetric HOMO-LUMO pair in the reactants. This is different from the ones in earlier exercise above where the HOMO-LUMO of the TS came from different pairs of reactant MOs combining. This is due to the MOs in the dienophile being lower due to the increased electron density from the oxygens. This means that this reaction has an inverse electron demand.
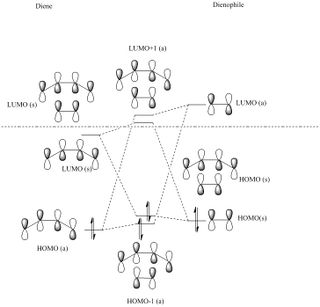
| Cyclohexadiene MO | 1,3-Dioxole MO | Resultant endo Transition State MO | Resultant exo Transition State MO |
|---|---|---|---|
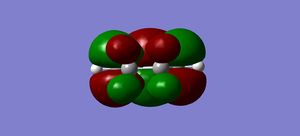
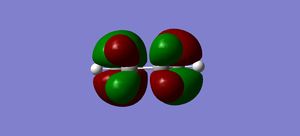 HOMO |
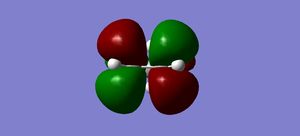 LUMO |
 HOMO-1 |
 HOMO-1 |
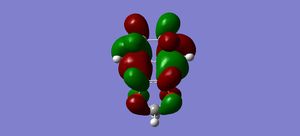
 LUMO+1 |
 LUMO+1 | ||
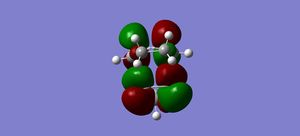
 LUMO |
 HOMO |
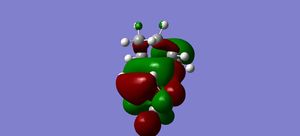 HOMO |
 HOMO |
 LUMO |
 LUMO |
Thermochemistry
| Reaction energy (Hartrees) | Reaction energy (KJ/mol) | Reaction barrier (Hartrees) | Reaction barrier (KJ/mol) | |
|---|---|---|---|---|
| Endo Diels-Alder | -0.034054 | -89.41 | +0.069928 | +183.60 |
| Exo Diels-Alder | -0.032724 | -85.92 | +0.072908 | +191.42 |
1 Hartree = 2625.499 kJ/mol
From the table it can seen clearly that the endo product is the kinetic and thermodynamic product in this reaction. The endo TS is of a lower energy than the exo TS hence it being the kinetic product and the endo product is also more stable by about 4 kJ/mol hence it being the thermodynamic product as well.
Secondary orbital interactions
Above it was mentioned that the endo TS is more stable, this is due to the secondary orbital interactions present in the endo TS. By inspection of the HOMO in the exo and endo it can be seen that there is a bonding interaction between p-orbital of the oxygen and the p-orbital of the diene in the endo and not in exo. This interaction makes the TS more stable than the exo TS. This secondary interaction is present due to the geometry of the molecule where in endo the reactants face the same way and has the oxygens above the diene whereas in exo the face opposite ways and the oxygen is not over/near the diene to interact.
Nf710 (talk) 10:41, 18 December 2017 (UTC) This section is good but quite brief again. It would have been good if you could have included a separate diagram showing SSO and also abit more detail on why the endo is the is the thermo product. Furthermore your energies are slightly out. Im not sure why perhaps you calculated the reaction energies etc with PM6.
Files
Exercise 3: Diels-Alder vs Cheletropic
Reaction overview
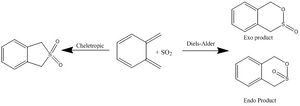
In this section the products of the reaction between SO2 and o-xylylene is probed. There are two types of reactions that could happen, Diels-Alder and Cheletropic.
Their transition states are looked at to see which has the highest reaction barrier and reaction energies, shown in the section. The transition states are optimized at the PM6 level as doing B3LYP would've been too taxing to be done in gaussview.
(Gaussview is only for visualisation. The calculations are performed in Gaussian Tam10 (talk) 15:18, 7 December 2017 (UTC))
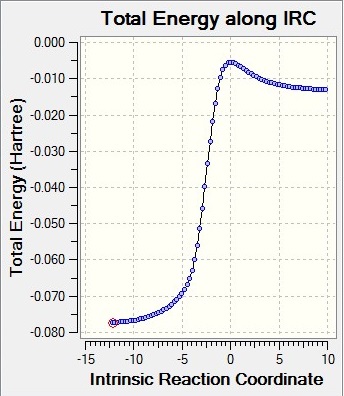
Intrinsic reaction coordinates
| endo Diels-Alder IRC | exo Diels-Alder IRC | Cheletropic IRC |
|---|---|---|
 |
 |

|
 |
 |

|
The table above shows the gif of the reaction taking place and the reaction coordinate. The reaction coordinate for endo is the wrong way around and the gif that it produces were reversed to put into the table.
It can be seen from this that the products of this reaction is much more stable than the reactants, this is because as the reaction proceeds the diene in the 6-membered ring turns into a triene (benzene) which obviously is aromatic and hence very stable.
Thermochemistry
Calculation
| Reaction energy (Hartrees) | Reaction energy (KJ/mol) | Reaction barrier (Hartrees) | Reaction barrier (KJ/mol) | |
|---|---|---|---|---|
| Endo Diels-Alder | -0.037506 | -98.47 | +0.031361 | +82.34 |
| Exo Diels-Alder | -0.037749 | -99.11 | +0.032873 | +86.31 |
| Cheletropic | -0.059201 | -155.43 | +0.039858 | +104.65 |
The above table shows the reaction barriers and energy of the reactions. This supports the IRCs shown earlier where the products are much more stable than the reactants with fairly accessible transition state.
Reaction profile diagram
The reaction profile diagram below shows that the cheletropic product is the most stable and hence the thermodyanamic product of the reaction between sulphur dioxide and o-xylylene but also that the transition state of this product is the one with the highest energy barrier. With the lowest activation energy the Endo product of the reaction is the kinetic product of the reaction but also the least stable of the three products. The Exo product has very similar reaction barrier and energy to the endo product and this can be understood from the IRCs above which shows that the approach of the reactants are very similar as well with just oxygen not part of the ring pointing out or in.

Files
Cheletropic Transition state IRC
Conclusion
From the course it can be seen that computational methods like this are very useful for understanding the transition structures 'present' in reactions. It helps to understand the theory behind why certain product is the dominant as opposed to the other. Also understand why a reaction wouldn't run in the lab. The only downside to this course is the relatively simple molecules that were analysed. To further understand how useful this computational method is more complex molecules in different reactions need to be analysed. It is very clear that Diels-Alder reactions and the one cheletropic one can be analysed thoroughly for the TS, reaction pathway, MO theory behind reaction.