Rep:Mod:dianaberheci
BH3 optimisation
Optimisation
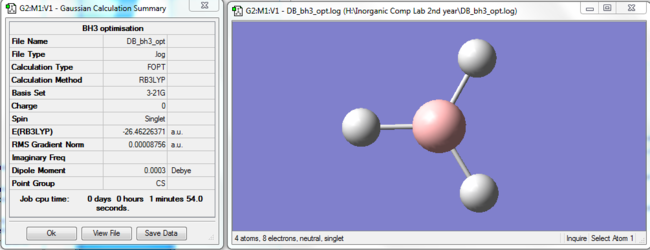
BH3 was optimised in Gaussian using the B3LYP method and 3-21G basis set. The results of the first optimisation are included in Figure 1.
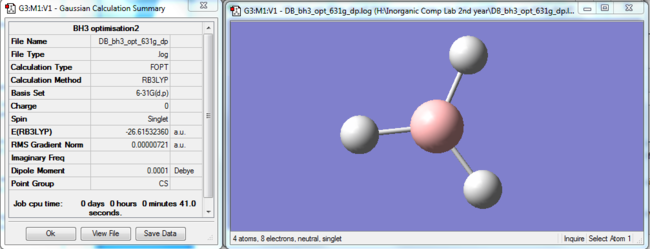
Further, a more detailed analysis was performed, using the same B3LYP method, but under the 6-31G(d,p) basis set, to yield the results in Figure 2.
 |
 |
The Item table for the 6-31G(d,p) optimisation can be seen below:
Item Value Threshold Converged?
Maximum Force 0.000217 0.000450 YES
RMS Force 0.000105 0.000300 YES
Maximum Displacement 0.000692 0.001800 YES
RMS Displacement 0.000441 0.001200 YES
Predicted change in Energy=-1.635269D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1947 -DE/DX = -0.0002 !
! R2 R(1,3) 1.1944 -DE/DX = -0.0001 !
! R3 R(1,4) 1.1948 -DE/DX = -0.0002 !
! A1 A(2,1,3) 119.9989 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0157 -DE/DX = 0.0 !
! A3 A(3,1,4) 119.9855 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
The frequency analysis gave the following results:
Low frequencies --- -2.2126 -1.0751 -0.0056 2.2359 10.2633 10.3194 Low frequencies --- 1162.9860 1213.1757 1213.1784
The link to the frequency log file can be found here: Db_bh3_freq.LOG
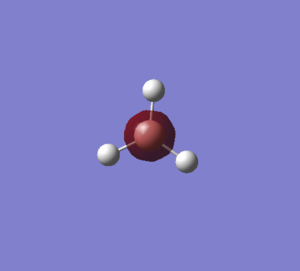
BH3 optimised molecule |
Vibrations analysis
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A1 | yes | out-of-plane bend |
| 1213 | 14 | E | very slight | bend |
| 1213 | 14 | E | very slight | bend |
| 2582 | 0 | A1 | no | symmetric stretch |
| 2715 | 126 | E | yes | asymmetric stretch |
| 2715 | 126 | E | yes | asymmetric stretch |
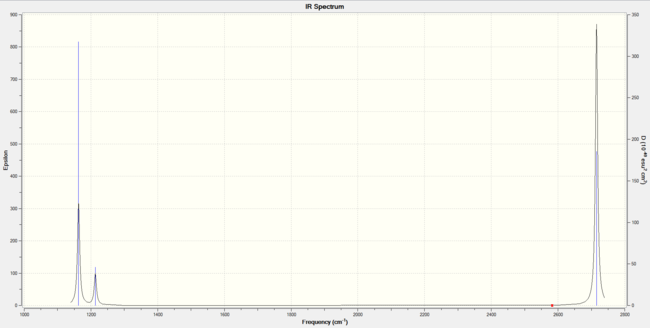 |
Although there are six vibrations for the BH3 molecule, not all of them are IR active due to some vibrations not fulfilling the IR selection rule. For a vibration to be IR active, there must be a change in dipole associated with the respective vibration [1]. The IR spectrum shows only 3 peaks, associated with the active vibration modes.
The active vibrations modes are the out-of-plane symmetric bend at 1163 cm-1, the 2 degenerate bond-angle deformation asymmetric bends at 1213 cm-1 and the 2 degenerate asymmetric stretches at 2715 cm-1. The only inactive vibration mode is the symmetric stretch, as this one does not bring about any dipole moment change.
Although it seems there are in fact 5 active vibration modes, there are 2 pairs of 2 degenerate vibrations, leading to only 3 peaks on the spectrum.
Smf115 (talk) 23:31, 22 May 2018 (BST)Very good explaination of the IR spectrum. The modes have been correctly assigned however, the symmetries aren't fully correct suggesting that your molecule wasn't of the correct point group for the frequnecy analysis calculation.
Molecular Orbitals analysis
The Molecular Orbitals of BH3 have been computed in Gaussian. The results have been compared with the Molecular Orbital Diagram obtained through LCAO, provided by Dr. Hunt (attached below).
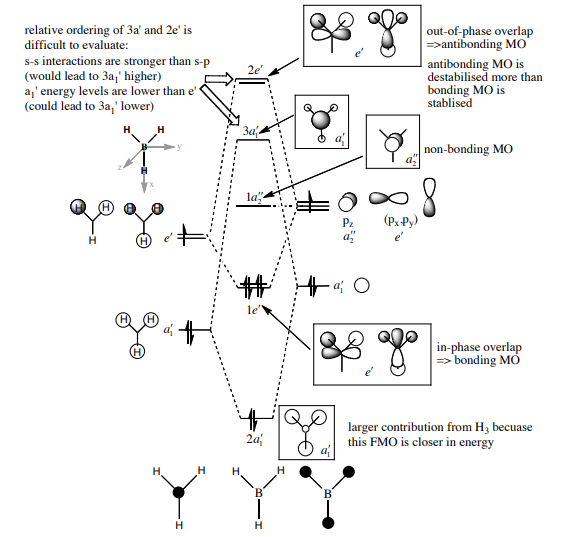 |
| Gaussian-computed Molecular Orbital snapshot | LCAO-obtained Molecular Orbital | Comparison |
|---|---|---|
 |
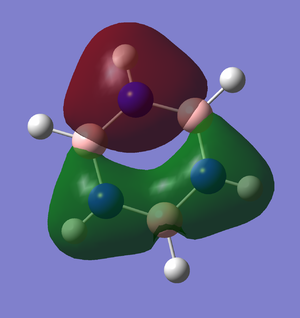
The first MO not shown on the LCAO-obtained MO diagram as it is not involved in bonding | The first MO of BH3 is very deep, therefore it won't be involved in bonding. The orbital is very close to the boron nucleus, as it is essentially arising from the 1s orbital of boron. |
 |
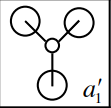 |
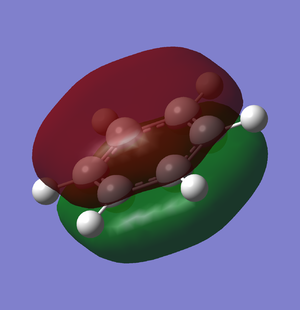
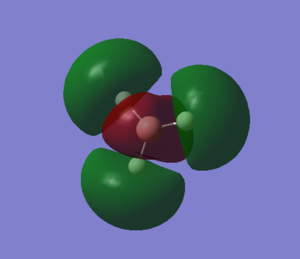
The second MO of BH3 is a sigma orbital which has an overall bonding character. It is formed by the in phase combination of the 2s orbital of boron and the H3 orbital with a1' symmetry. Gaussian and LCAO method both give very similarly shaped MOs. |
 |
 |
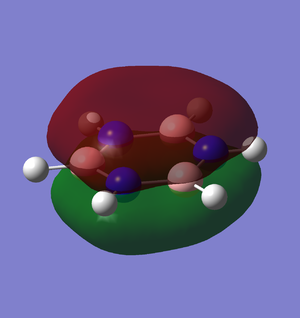
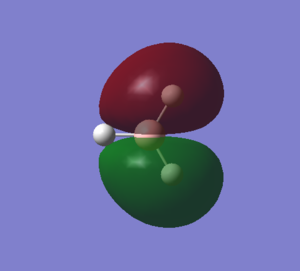
The third MO arises from the sigma in-phase interaction between the 2py orbital of boron and one of the e' H3 orbitals. The MO is higher in energy than the previous one as it has got a nodal plane along the x axis. The 2 representations are again fairly similar, but it is important to note that Gaussian gives a more accurate picture of the MO, as it accounts for the final MO shape resulting from the combination of the FMOs: the resulting MO is more dense towards the hydrogen atoms and less dense towards the other hydrogen on the x axis. |
 |
 |
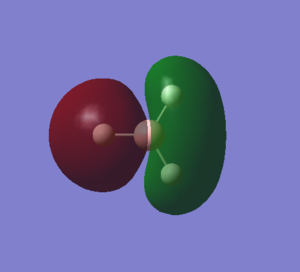
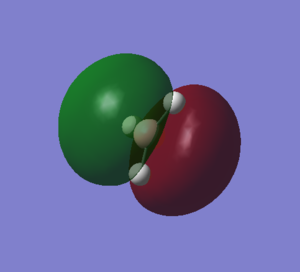
This MO arises from the in-phase interactions between the 2px of boron and the other e' of H3. There is a nodal plane along the z axis now. The 2 representations are fairly similar, but the Gaussian one is again more representative for the real situation, as it accounts for the in-phase interactions between the 2H and the green lobe of the p orbital. |
 |
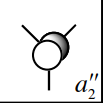 |
The fifth MO is the non-bonding MO of BH3, as the pz orbital does not have the right symmetry to interact with any of the H3 orbitals. Whilst both pictures are similar, the Gaussian image accounts for the large size of this orbital and the extension of its surface towards the B-H bonds. |
 |
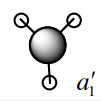 |
The sixth MO is the LUMO and it is formed from the out-of-phase interactions between the 2s of boron and the a1' symmetry orbital from H3, leading to an anti-bonding MO. |
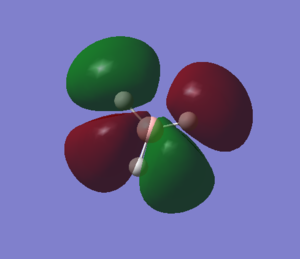 |
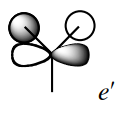 |
The seventh MO looks slightly different in the 2 representations, as in the Gaussian model, the orbital electron density is extended towards the x axis as well. The interactions are again out-of-phase, leading to an anti-bonding orbital. |
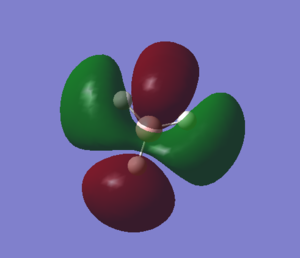 |
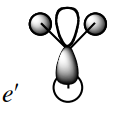 |
This MO does not have the exact same shape as described by the LCAO, as the shapes of the orbitals making up the MO change upon forming the MO. This MO is composed of out-of-phase interactions, and it is an anti-bonding one. |
Smf115 (talk) 23:33, 22 May 2018 (BST)Thorough and well presented comparison of the calculated MOs and the corresponding LCAOs.
NH3 optimisation
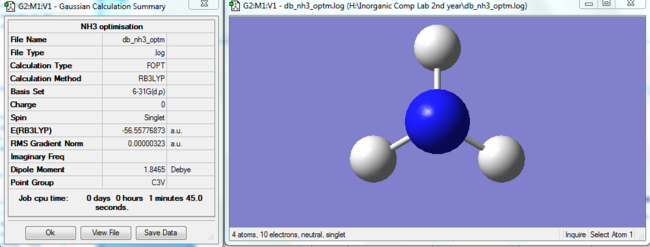 |
The Item table can be seen below:
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000012 0.001800 YES
RMS Displacement 0.000008 0.001200 YES
Predicted change in Energy=-9.844604D-11
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7446 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7446 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7446 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8637 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
The link to the frequency log file can be found here: DB NH3 FREQ.LOG
Low frequencies --- -8.5646 -8.5588 -0.0047 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
NH3BH3 optimisation
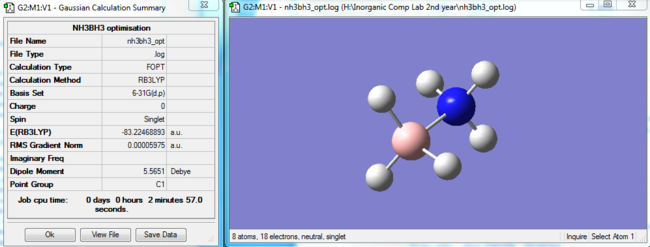 |
The Item table can be seen below:
Item Value Threshold Converged?
Maximum Force 0.000122 0.000450 YES
RMS Force 0.000058 0.000300 YES
Maximum Displacement 0.000513 0.001800 YES
RMS Displacement 0.000296 0.001200 YES
Predicted change in Energy=-1.631175D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,7) 1.21 -DE/DX = -0.0001 !
! R2 R(2,7) 1.21 -DE/DX = -0.0001 !
! R3 R(3,7) 1.21 -DE/DX = -0.0001 !
! R4 R(4,8) 1.0186 -DE/DX = -0.0001 !
! R5 R(5,8) 1.0186 -DE/DX = -0.0001 !
! R6 R(6,8) 1.0186 -DE/DX = -0.0001 !
! R7 R(7,8) 1.6681 -DE/DX = -0.0001 !
! A1 A(1,7,2) 113.8743 -DE/DX = 0.0 !
! A2 A(1,7,3) 113.8743 -DE/DX = 0.0 !
! A3 A(1,7,8) 104.5972 -DE/DX = 0.0 !
! A4 A(2,7,3) 113.874 -DE/DX = 0.0 !
! A5 A(2,7,8) 104.5968 -DE/DX = 0.0 !
! A6 A(3,7,8) 104.5969 -DE/DX = 0.0 !
! A7 A(4,8,5) 107.8685 -DE/DX = 0.0 !
! A8 A(4,8,6) 107.8685 -DE/DX = 0.0 !
! A9 A(4,8,7) 111.0294 -DE/DX = 0.0 !
! A10 A(5,8,6) 107.8686 -DE/DX = 0.0 !
! A11 A(5,8,7) 111.0303 -DE/DX = 0.0 !
! A12 A(6,8,7) 111.0302 -DE/DX = 0.0 !
! D1 D(1,7,8,4) 179.9998 -DE/DX = 0.0 !
! D2 D(1,7,8,5) -60.0003 -DE/DX = 0.0 !
! D3 D(1,7,8,6) 60.0001 -DE/DX = 0.0 !
! D4 D(2,7,8,4) -60.0 -DE/DX = 0.0 !
! D5 D(2,7,8,5) 59.9998 -DE/DX = 0.0 !
! D6 D(2,7,8,6) -179.9998 -DE/DX = 0.0 !
! D7 D(3,7,8,4) 59.9997 -DE/DX = 0.0 !
! D8 D(3,7,8,5) 179.9995 -DE/DX = 0.0 !
! D9 D(3,7,8,6) -60.0001 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
The link to the frequency log file can be found here: Db NH3BH3 FREQ.LOG
Low frequencies --- -0.0007 0.0009 0.0015 13.6144 18.8476 42.5238 Low frequencies --- 266.2562 632.2749 639.0335
Association Energy
ENH3=-56.55776 a.u.
EBH3=-26.61532 a.u.
ENH3BH3=-83.22468 a.u.
The dissociation energy of the B-N covalent bond is 389 kJ/mol [3] (thus, -389 kJ/mol for association). However, the bond energy between B and N in NH3BH3 is about a third of the literature value, meaning that the bond strength between B and N in this compound is much weaker. This fits the theory model well, as the B-N bond here is not a simple covalent bond, it's a dative covalent bond formed by donation of a lone pair of electrons from nitrogen to an empty p orbital of boron. Indeed, the literature value for a dative B-N bond is [3], which is very close to the one obtained from the Gaussian calculations, thus confirming the nature of the B-N bond in NH3BH3. The dative bond is rather weak compared to a simple covalent bond because upon heterolytic breaking, the bond yields 2 stable compounds, ammonia and borane.
BBr3 optimisation
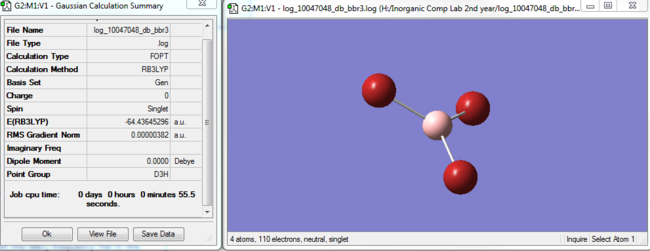 |
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000036 0.001800 YES
RMS Displacement 0.000023 0.001200 YES
Predicted change in Energy=-4.026879D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.934 -DE/DX = 0.0 !
! R2 R(1,3) 1.934 -DE/DX = 0.0 !
! R3 R(1,4) 1.934 -DE/DX = 0.0 !
! A1 A(2,1,3) 120.0 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0 -DE/DX = 0.0 !
! A3 A(3,1,4) 120.0 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
The link to the frequency log file can be found here: Db bbr3 log.LOG
Low frequencies --- -0.0116 -0.0065 -0.0004 49.9507 49.9507 50.0316 Low frequencies --- 144.7605 144.7639 215.6179
BBr3 optimised molecule |
The link to the DOI of the analysis for this molecule can be found here: DOI:10042/202358
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 145 | 0.09 | E | no | bend |
| 145 | 0.09 | E | no | bend |
| 216 | 0 | A1 | no | symmetric stretch |
| 407 | 5 | A1 | slightly | out-of-plane bend |
| 622 | 313 | E | yes | asymmetric stretch |
| 622 | 313 | E | yes | asymmetric stretch |
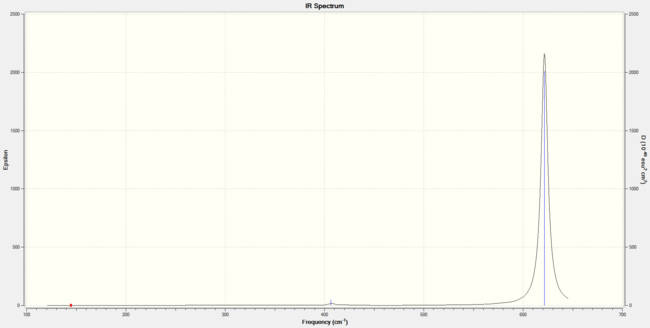 |
Compared to BH3, BBr3 will show vibrations of much lower frequency, giving less peaks in the IR spectrum due to the small intensities of the respective vibrations. This is because bromine atoms are much heavier than hydrogen atoms, and the molecular weight of the molecule increases significantly, thus reducing the vibration frequencies.
Smf115 (talk) 23:33, 22 May 2018 (BST)Nice additional comparsion of the BH3 and BBr3 IR spectra.
Project: Aromaticity
Benzene analysis
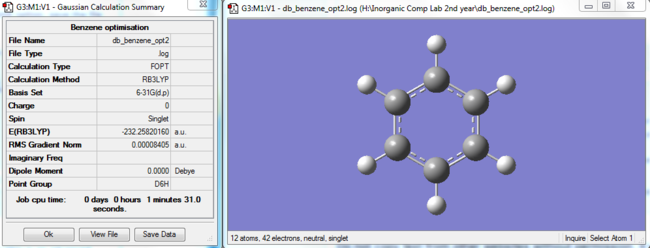 |
Item Value Threshold Converged?
Maximum Force 0.000194 0.000450 YES
RMS Force 0.000077 0.000300 YES
Maximum Displacement 0.000824 0.001800 YES
RMS Displacement 0.000289 0.001200 YES
Predicted change in Energy=-4.246203D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.3962 -DE/DX = 0.0001 !
! R2 R(1,6) 1.3962 -DE/DX = 0.0001 !
! R3 R(1,7) 1.0861 -DE/DX = 0.0002 !
! R4 R(2,3) 1.3962 -DE/DX = 0.0001 !
! R5 R(2,8) 1.0861 -DE/DX = 0.0002 !
! R6 R(3,4) 1.3962 -DE/DX = 0.0001 !
! R7 R(3,9) 1.0861 -DE/DX = 0.0002 !
! R8 R(4,5) 1.3962 -DE/DX = 0.0001 !
! R9 R(4,10) 1.0861 -DE/DX = 0.0002 !
! R10 R(5,6) 1.3962 -DE/DX = 0.0001 !
! R11 R(5,11) 1.0861 -DE/DX = 0.0002 !
! R12 R(6,12) 1.0861 -DE/DX = 0.0002 !
! A1 A(2,1,6) 120.0 -DE/DX = 0.0 !
! A2 A(2,1,7) 120.0 -DE/DX = 0.0 !
! A3 A(6,1,7) 120.0 -DE/DX = 0.0 !
! A4 A(1,2,3) 120.0 -DE/DX = 0.0 !
! A5 A(1,2,8) 120.0 -DE/DX = 0.0 !
! A6 A(3,2,8) 120.0 -DE/DX = 0.0 !
! A7 A(2,3,4) 120.0 -DE/DX = 0.0 !
! A8 A(2,3,9) 120.0 -DE/DX = 0.0 !
! A9 A(4,3,9) 120.0 -DE/DX = 0.0 !
! A10 A(3,4,5) 120.0 -DE/DX = 0.0 !
! A11 A(3,4,10) 120.0 -DE/DX = 0.0 !
! A12 A(5,4,10) 120.0 -DE/DX = 0.0 !
! A13 A(4,5,6) 120.0 -DE/DX = 0.0 !
! A14 A(4,5,11) 120.0 -DE/DX = 0.0 !
! A15 A(6,5,11) 120.0 -DE/DX = 0.0 !
! A16 A(1,6,5) 120.0 -DE/DX = 0.0 !
! A17 A(1,6,12) 120.0 -DE/DX = 0.0 !
! A18 A(5,6,12) 120.0 -DE/DX = 0.0 !
! D1 D(6,1,2,3) 0.0 -DE/DX = 0.0 !
! D2 D(6,1,2,8) 180.0 -DE/DX = 0.0 !
! D3 D(7,1,2,3) 180.0 -DE/DX = 0.0 !
! D4 D(7,1,2,8) 0.0 -DE/DX = 0.0 !
! D5 D(2,1,6,5) 0.0 -DE/DX = 0.0 !
! D6 D(2,1,6,12) 180.0 -DE/DX = 0.0 !
! D7 D(7,1,6,5) 180.0 -DE/DX = 0.0 !
! D8 D(7,1,6,12) 0.0 -DE/DX = 0.0 !
! D9 D(1,2,3,4) 0.0 -DE/DX = 0.0 !
! D10 D(1,2,3,9) 180.0 -DE/DX = 0.0 !
! D11 D(8,2,3,4) 180.0 -DE/DX = 0.0 !
! D12 D(8,2,3,9) 0.0 -DE/DX = 0.0 !
! D13 D(2,3,4,5) 0.0 -DE/DX = 0.0 !
! D14 D(2,3,4,10) 180.0 -DE/DX = 0.0 !
! D15 D(9,3,4,5) 180.0 -DE/DX = 0.0 !
! D16 D(9,3,4,10) 0.0 -DE/DX = 0.0 !
! D17 D(3,4,5,6) 0.0 -DE/DX = 0.0 !
! D18 D(3,4,5,11) 180.0 -DE/DX = 0.0 !
! D19 D(10,4,5,6) 180.0 -DE/DX = 0.0 !
! D20 D(10,4,5,11) 0.0 -DE/DX = 0.0 !
! D21 D(4,5,6,1) 0.0 -DE/DX = 0.0 !
! D22 D(4,5,6,12) 180.0 -DE/DX = 0.0 !
! D23 D(11,5,6,1) 180.0 -DE/DX = 0.0 !
! D24 D(11,5,6,12) 0.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
The link to the frequency log file can be found here: benzene
Low frequencies --- -2.1456 -2.1456 -0.0088 -0.0042 -0.0041 10.4835 Low frequencies --- 413.9768 413.9768 621.1390
Benzene optimised molecule |
Borazine analysis
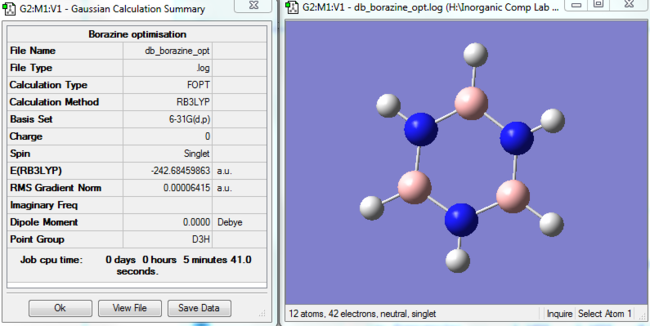 |
Item Value Threshold Converged?
Maximum Force 0.000085 0.000450 YES
RMS Force 0.000033 0.000300 YES
Maximum Displacement 0.000251 0.001800 YES
RMS Displacement 0.000075 0.001200 YES
Predicted change in Energy=-9.309158D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,9) 1.0098 -DE/DX = 0.0 !
! R2 R(2,11) 1.1949 -DE/DX = 0.0001 !
! R3 R(3,8) 1.0098 -DE/DX = 0.0 !
! R4 R(4,10) 1.1949 -DE/DX = 0.0001 !
! R5 R(5,7) 1.0098 -DE/DX = 0.0 !
! R6 R(6,12) 1.1949 -DE/DX = 0.0001 !
! R7 R(7,10) 1.4307 -DE/DX = -0.0001 !
! R8 R(7,12) 1.4307 -DE/DX = -0.0001 !
! R9 R(8,10) 1.4307 -DE/DX = -0.0001 !
! R10 R(8,11) 1.4307 -DE/DX = -0.0001 !
! R11 R(9,11) 1.4307 -DE/DX = -0.0001 !
! R12 R(9,12) 1.4307 -DE/DX = -0.0001 !
! A1 A(5,7,10) 118.5609 -DE/DX = 0.0 !
! A2 A(5,7,12) 118.5609 -DE/DX = 0.0 !
! A3 A(10,7,12) 122.8782 -DE/DX = 0.0 !
! A4 A(3,8,10) 118.5609 -DE/DX = 0.0 !
! A5 A(3,8,11) 118.5609 -DE/DX = 0.0 !
! A6 A(10,8,11) 122.8782 -DE/DX = 0.0 !
! A7 A(1,9,11) 118.5609 -DE/DX = 0.0 !
! A8 A(1,9,12) 118.5609 -DE/DX = 0.0 !
! A9 A(11,9,12) 122.8782 -DE/DX = 0.0 !
! A10 A(4,10,7) 121.4391 -DE/DX = 0.0 !
! A11 A(4,10,8) 121.4391 -DE/DX = 0.0 !
! A12 A(7,10,8) 117.1218 -DE/DX = 0.0 !
! A13 A(2,11,8) 121.4391 -DE/DX = 0.0 !
! A14 A(2,11,9) 121.4391 -DE/DX = 0.0 !
! A15 A(8,11,9) 117.1218 -DE/DX = 0.0 !
! A16 A(6,12,7) 121.4391 -DE/DX = 0.0 !
! A17 A(6,12,9) 121.4391 -DE/DX = 0.0 !
! A18 A(7,12,9) 117.1218 -DE/DX = 0.0 !
! D1 D(5,7,10,4) 0.0 -DE/DX = 0.0 !
! D2 D(5,7,10,8) 180.0 -DE/DX = 0.0 !
! D3 D(12,7,10,4) 180.0 -DE/DX = 0.0 !
! D4 D(12,7,10,8) 0.0 -DE/DX = 0.0 !
! D5 D(5,7,12,6) 0.0 -DE/DX = 0.0 !
! D6 D(5,7,12,9) 180.0 -DE/DX = 0.0 !
! D7 D(10,7,12,6) 180.0 -DE/DX = 0.0 !
! D8 D(10,7,12,9) 0.0 -DE/DX = 0.0 !
! D9 D(3,8,10,4) 0.0 -DE/DX = 0.0 !
! D10 D(3,8,10,7) 180.0 -DE/DX = 0.0 !
! D11 D(11,8,10,4) 180.0 -DE/DX = 0.0 !
! D12 D(11,8,10,7) 0.0 -DE/DX = 0.0 !
! D13 D(3,8,11,2) 0.0 -DE/DX = 0.0 !
! D14 D(3,8,11,9) 180.0 -DE/DX = 0.0 !
! D15 D(10,8,11,2) 180.0 -DE/DX = 0.0 !
! D16 D(10,8,11,9) 0.0 -DE/DX = 0.0 !
! D17 D(1,9,11,2) 0.0 -DE/DX = 0.0 !
! D18 D(1,9,11,8) 180.0 -DE/DX = 0.0 !
! D19 D(12,9,11,2) 180.0 -DE/DX = 0.0 !
! D20 D(12,9,11,8) 0.0 -DE/DX = 0.0 !
! D21 D(1,9,12,6) 0.0 -DE/DX = 0.0 !
! D22 D(1,9,12,7) 180.0 -DE/DX = 0.0 !
! D23 D(11,9,12,6) 180.0 -DE/DX = 0.0 !
! D24 D(11,9,12,7) 0.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
The link to the frequency log file can be found here: borazine
Low frequencies --- -6.5952 -6.4169 -5.9633 -0.0097 0.0576 0.1409 Low frequencies --- 289.2513 289.2617 403.8549
Borazine optimised molecule |
Charge Distribution Comparison
 |
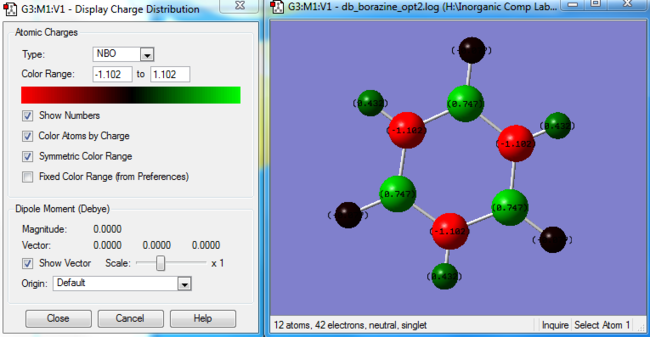 |
| Molecule | Atom | Charge |
| Benzene | Carbon (all 6 carbons identical) | -0.239 |
| Benzene | Hydrogen (all 6 hydrogens identical) | 0.239 |
| Borazine | Boron (all 3 borons identical) | 0.747 |
| Borazine | Nitrogen (all 3 nitrogens identical) | -1.102 |
| Borazine | Hydrogens connected to boron atoms (3 hydrogens) | -0.077 |
| Borazine | Hydrogens connected to nitrogen atoms (3 hydrogens) | 0.432 |
In benzene, the ring is formed by six identical carbons, each connected to one hydrogen. Therefore, the molecule will not have a varied charge distribution. As carbon is slightly more electronegative than hydrogen, it will have a slightly higher electron density than hydrogen. However, borazine, although having a zero dipole moment, shows a more complicated charge distribution. This arises as a consequence of the differences in electronegativities between nitrogen, hydrogen and boron (nitrogen is the most electronegative, followed by hydrogen and boron). Therefore, the N-H bonds will be polarised towards nitrogen (which will have a more negative charge), whereas the B-H bonds will be polarised towards hydrogen, leaving the borons to be more electron deficient.
Molecular Orbitals Comparison
Smf115 (talk) 23:47, 22 May 2018 (BST)Nice MO analsysis with some key terminology and corresponding MOs selected by shape. However, it would have been better to see a larger range of character covered by the MOs chosen and more details included, such as pi-type interactions for MO 17, to compare/describe the MOs more effectively.
Aromaticity concepts
Usually, aromaticity is a concept applying to cyclic, planar, conjugated systems which obey Huckel's rule of 4n+2 pi electrons. This rule states that aromatic compounds must have 4n+2 pi delocalised electrons. [4] Aromatic compounds are much more stable than equivalent compounds exhibiting localised double bonds and they do not usually undergo electrophilic additions to the double bonds; instead, they are subject to aromatic electrophilic substitutions.
In classic compounds, such as benzene, the origin of aromaticity is related to the overlap of the pz orbitals from each carbon involved in the conjugated system. For example, in benzene, each carbon atom is sp2 hybridised, which means each of the carbons has a pz orbital perpendicular to the ring plane. The 6 pz atomic orbitals will overlap, creating regions of delocalised electrons above and below the plane of the benzene ring.
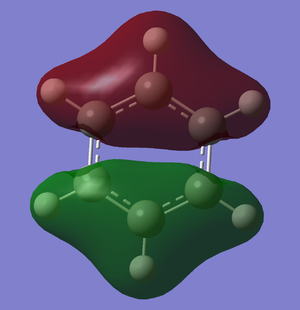
A better picture of the aromaticity in benzene is given by the molecular orbitals of benzene (see Figure x). Thus, it can be seen that the lowest molecular orbital π1 has got the highest bonding character, while following 2 have a weaker bonding character. These 3 bonding molecular orbitals will all be completely filled, and the last 3 molecular orbitals, the anti-bonding ones, will be empty. This confers a very high stability to benzene, as it has got the maximal stabilisation it can achieve, as all its bonding molecular orbitals filled with electron pairs.
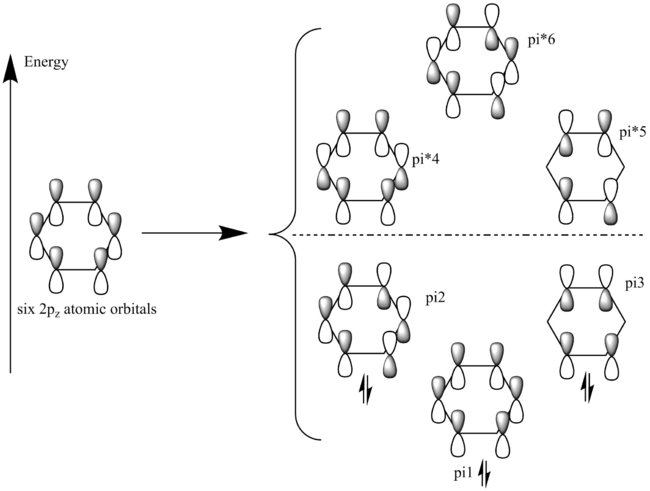 |
Apart from the ability of the π orbitals to accommodate all the electrons exclusively in the bonding orbitals, the σ-framework of the benzene (formed by the sp2 atomic orbitals of each carbon) contributes to the stability, as the σ bonds arrange in a hexagon shape, which allows for a perfect C-C-C bond angle of 120 degrees, therefore the σ framework does not suffer of any strain. These features add up to the stability of the aromatic systems. [5]
Smf115 (talk) 23:44, 22 May 2018 (BST)Good understanding of the LCAO approach for the traditional overlapping pZ AOs in benzene shown. To improve though consider the MOs visualised for benzene and how the delocalisation of the electron density is due to a number of the MOs and not just MO 17 pictured above (including sigma-type interactions). Developing the discussion of aromaticity from just Huckel's rule to include other criteria would also be good to see.
Smf115 (talk) 23:51, 22 May 2018 (BST)Overall a good report with a strong first section, the discussion sections in the project section just required a bit more development.
References
- ↑ J.M. Brown, Molecular Spectroscopy, Oxford University Press, 1998
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Dr. P. Hunt, Molecular Orbitals in Inorganic Chemistry 2nd year UG course, Imperial College London, November, 2017
- ↑ 3.0 3.1 A. Haaland, Anxrw. Chem. Inl. Ed. Engl., 1989, 28, 992-1007
- ↑ M. Sainsbury, Aromatic Chemistry, Oxford University Press, 1992
- ↑ P. Atkins, J. de Paula, Atkins' Physical Chemistry, Oxford University Press, 2014, 894-902