Rep:Mod:demo project
Mini Project-Borane structures
In this mini-project boranes and specfiically borane clusters will be investigated into. Firstly the general electronic structures of some closo boranes will be looked at. Next specific types of carboranes and their function as superacids will be investigated. The last thing that will be looked at is a specific reaction of a closo-borane and the electronic structure of the product of the reaction.
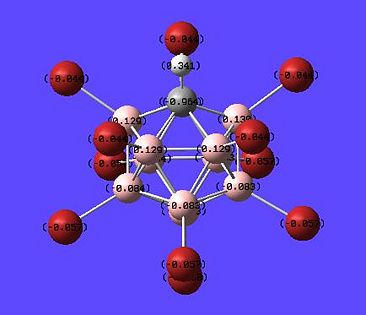
The inital molecule that is going to be investigated is the Closo-B6H62-. This was drawn in Chembio3D, saved as a .gjf and then opened in gaussview. The molecule was initally drawn such that it was in an octahedral geometry as predicted by Wade's rules. Then a DFT, B3LYP, 6-31(d,p) optimisation and frequency calculation was run on the molecule. The molecule also had a 2- charge added to it. This was done by editing the script in the following way (note the -2 just above the atomic co-ordinates specifying a charge and the pop=full for a full MO analysis):
# opt freq b3lyp/6-31g(d,p) pop=full b2h6opt_freq dft 6-21g -2 1 B -3.86600700 1.16137100 -1.34015600 etc...
The resulting molecule co-ordinates can be seen below:
Closo-B6H62- DOI:10042/to-1054
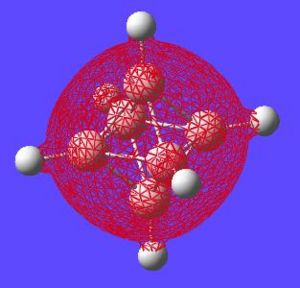
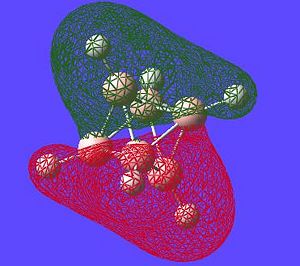
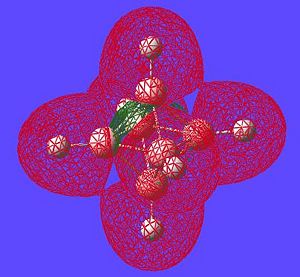
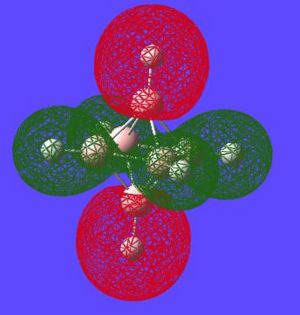
The next thing that is interesting to look at is the generated MO's of the molecule. [1] Closo-boranes tend to be very unreactive compared to other types of boron cluster (nido-, arachno-). This stability has been suggested to be linked to "3-dimensional aromaticity". The MO diagram of this cluster can be looked at in order to try and justify such an aromatic type system.
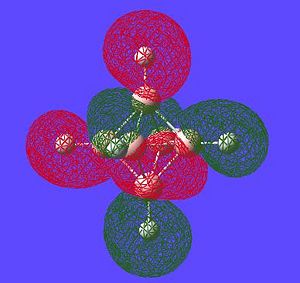
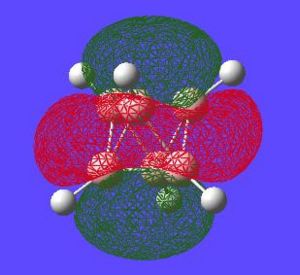
Aromaticity is an extra stabilisation recognised in a molecule that is not accounted for conjugation alone and can be thought of as a cylic delocalisation of electrons.[2]. Thus if in a molecular orbital there looks to be a possibility of this kind of cyclic (or spherical in three dimensions) delocalisation of electrons this could lead to aromaticityin the molecule. After opening and looking through the molecular orbitals of this struture these are the orbitals that look like they could help lead to aromaticity:
Here are the molecular orbitals that look like they should give rise to some sort of aromatic stability:
Note: the orbitals calculated can be imagined with a smaller isovalue such that some lobes that wouldnt usually meet, would meet due to the "increase in size" of the orbitals boundary surface
| Potential Aromatic MOs of Closo-B6H62- | ||
|---|---|---|
|
|
|
|
|
|
Another interesting point to make is that all occupied orbtials are bonding in the case of this cluster and no antibonding are occupied[1]. This is just like a benzene type arrangement where all the occupied orbitals lie below the non-bonding level. Looking at the evidence the 3D aromaticity of Closo-B6H62- seems likely.
Now to look at the potential superacidic properties of related carborane species. Taking the closo-B12H122- borane cluster and replacing one of the boranes with a carbon (and then reducing the overall charge to -1 to maintain the closo structure), and replacing all boron hydrogens with chlorine results in the conjugate base (anion) of a superacid (H(CHB11Cl11). This superacid is one million times stronger than sulphuric acid and can be used to protonate C60 without damaging or decomposing it. In order for this to be a strong acid the conjugate carbonane anion must be highly stable. This might be expected given the 3D aromatic nature of borane/carborane clusters that would help delocalise the charge slightly. Also electronegative substituents can be used to aid the stability of the resulting anion.
In order to study this stability an acid base reaction scheme will be studied. This reaction scheme that will be used is:
H(CHB11Cl11) + H2O --> (CHB11Cl11)-1 + H3O+
So protons will be used as the acid of carborane base (in reality a variety of Lewis acids can be used) and since a proton can't be modelled electronically (no electrons) water and the hydronium are introduced into the reaction scheme.
Water and hydronium are modelled at the same level as the carborane anion base and the protonated carbonane acid. This resulted in energies for the two species of:
H2O = -76.41431631 Hartree
H3O+ = -76.70417459 Hartree
So the energy gain from protonating water is 761.02 kJmol-1
The superacid will also be modified in order to see if the acidity of the superacid can be changed. This will be done by changing the EWG groups attached to the acid and by changing the number of EWG groups attached. The electronegative Cl atoms will be changed to F and Br and the effect will be noted. Also the structure will be tested with only 1, 2 and 3 Chlorines attached and seeing how the acidity is effected by this. In order to run the calculations (optimisation, frequency) in the cases where the types of atoms will be changed the Lanl2dz basis set will be used with the b3lyp DFT method. The Lanl2dz method is used to account for the heavier atoms in some of the structures (e.g. Br) by using pseudopotentials for the non-valence electrons. Obviously if this method/basis set if used for the heavier elements (Br) for fair comparison to idenfify a trend it must also be used when only first row (F), and first and second row (Cl) elements are present also. When looking at changing the number of electronegative elements only Chlorine (nothing heavier) is used and so the 6-31g(d,p) basis set will be used to run the calculations. Note also it is important to ensure the charge is correct in each case (-1 for anion and 0 for protonated species). This can be done directly in gaussview under calculate-->gaussian-->method-->charge or can be done by directly editing the script. In the script below the -1 refers to the charge and the 1 to the multiplicity (singlet state).
# opt freq b3lyp/6-31g(d,p) pop=full [No Title] -1 1 B -2.44622600 -0.19667600 -0.09126900 B -3.37917500 0.16858900 1.28593600 B -4.88760500 -0.60752500 1.12206200
Here is the script used for the "changing atom type" calculations:
# opt freq b3lyp/lanl2dz pop=full
Here is the script used for the "changing number of substituents" calculations:
# opt freq b3lyp/6-31g(d,p) pop=full
Another thing that must be considered is the site of protonation of the carbanion in the protonated version (i.e. where would it protonate if it had to?). In order to do this the optimised standard (i.e. all borons with chlorines attached) superacid was run through an NBO charge analysis. This is run using the following:
# rb3lyp/lanl2dz pop=nbo geom=connectivity
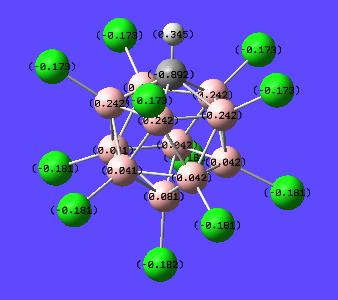
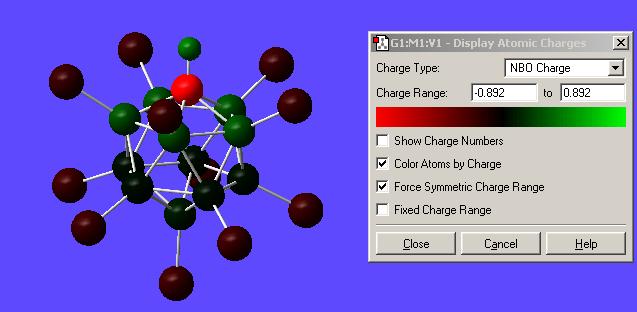
Once the calculation was run and the output file opened the charges were displayed by going Results-->Charges--> and then selecting NBO from the "charge type" drop down menu. Here is the result of this calculation with numerical values and with a "colour by charge" scheme (see image for colour-to-charge spectrum):
Chlorinated NBO DOI:10042/to-1073
| Charge Distribution on Superacid's conjugate base | |
|---|---|
|
|
This has shown that the excess extra electron is found mainly on the carbon (-0.892). However the chlorines also help stabilise this extra charge (-0.173 and -0.181). It is expected that the carbon would have this extra charge situated mainly on it since it is group 4 rather than group 3 (Boron). This then means if one was to protonate (or add a lewis acid to) the structure it would be expected to sit on this excess negative charge. Thus the proton was placed onto the carbon alongside the other hydrogen and the optimisation-frequency calculations run again in each case.
Here are the resultant found structures for the superacids and there conjugate base: Order: Fully Chlorinated, Fully Fluorinated, Fully Brominated, Singly Chlorinated, Doubly Chlorinated, Triply Chlorinated
| Superacids optimised structures | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Superacids | ||||||||||||||||||
| Coresponding carbanion | ||||||||||||||||||
Anion Publications (In order of left to right): Unable to publish,DOI:10042/to-1077 , Unable to publish,DOI:10042/to-1078 ,DOI:10042/to-1079 ,DOI:10042/to-1080 .
Superacid (In order of left to right): DOI:10042/to-1076 , Unable to publish, Unable to publish,DOI:10042/to-1074 ,DOI:10042/to-1075 , Unable to Publish.
Here are the energy results for the carborane anions (second energy) as well as their protonated counterparts (first energy):
all fluorine: -1411.47931514 -1411.18417848 difference=0.29513666 Hartree= 774.8813 kJmol-1 overall reaction change: 13.8584kJmol-1
all chlorine: -477.38454386 -477.09255566 difference=0.2919882Hartree= 766.6150 kJmol-1 overall reactio change: 5.5921kJmol-1
all bromine: -457.74642850 -457.43741447 difference=0.30901403Hartree= 811.3163 kJmol-1 overall reaction change: 50.2934kJmol-1
1 chlorine: -779.01767504 -778.64912229 difference=0.36855275Hartree= 967.6352 kJmol-1 overall reaction change: 206.6152kJmol-1
2 chlorine: -1238.2905830 -1238.65631532 difference=0.36573232Hartree= 960.2302 kJmol-1 overall reaction: 199.21kKmol-1
3 chlorine: -1698.2908 -1697.924055 difference=0.366745Hartree= 962.8890 kJmol-1 overall reaction change of 201.8690kJmol-1.
These results are extremely good and some analysis can now be done on the numbers. The most acidic molecule when modifying the different atoms attached is that with all chlorines attached. One might expect that the flourinated acid would be the most stable because of the fact it is the most electronegative and would help stabilise the anion chrage the best on deprotonation. This is however not the case. The superacid is least acidic following the electronegativity trend (least electronegative least help in stabilising charge and so least acidic). In order to help confirm that one would expect F to help stabilise the charge best NBO charge calculations are run on the two remaining molecules (Br and F). If indeed the F should stabilise the charge the best there must be another effect thst is causing it to be the least acidic of the set.
The absolute values of these reaction energies can be interpreted by comparing to the thermal energy available at room temperature (298.15K). Energy available=RT=2.45kJmol-1. Thus given the energy in the calculations can be up to 5kJmol-1 out the energies for the flourine could be quite low also. Including this energy error in the Cl case the energy could be well under this thermal energy (could even be below 0) and would explain the high acidity. For the bromine case the reaction energy would still be very high even considering this error and so wouldn;t be expected to be a very good acid compared to the other two. Another good comparison to make is to a hydrogen bond. A hydrogen bond is consdiered a weak interaction with energies varying from around 5-30kJmol-1[3]. A weak covalent bond is also around 155kJmol-1 and a normal C-H bond around 413kJmol-1 [4]. Thus this loss of a proton process is alot weaker than even in a "weak" hydrogen bond. Thus the fully chlorinated superacids acidity can be compared to something tangible and can be recognised for the strong acid it is using computational methods.
All this is of course assuming a thermodynamic arguement. In order to get kinetic information out the transition states would have to be studied. One might expect, roughly, using hammonds postulate that the most important factor in stanilising the transition state is the charge stabilisation but this would have to properly studied to be conclusive.
In terms of varying the number of chlorines attached the trend is generally as expected. Clearly having only 1-3 chlorines attached has caused it to be far less acidic than when all Borons have chlorines on with an overall energy of reaction of 206.6152kJmol-1 (cf. 5.5921kJmol-1). However increasing the number of chlorines does increase the acidity of the superacid as expected generally; more electronegative elements help stabilise the charge of the anion and so increase the acidity of the superacid. However just over the ran 1Cl, 2Cl and 3Cl experiments although going from 1 to 3 chlorines the acidity increases but going from 1-2 it decreases. In fact having just two Chlorines is the most acidic of the three situations. The errors of the calculated energies is likely to be enough such that the energies overlap and so increasing number of chlorine does increase the acidity and the error of the experiment leads one to believe otherwise OR On a large scale trend adding chlorines does increase the acidity of the superacid but over the range of a 1 chlorine increase subtle orbital effects may not mean there is always an increase on adding each carbon.
So lets look at the results of the NBO analysis of the F and Br substituted superacids and see of any light can be shone on the above results.
Fluorinated superacid NBO DOI:10042/to-1072
Brominated superacid NBO DOI:10042/to-1071
| Fluorinated Superacid | Brominated Superacid |
|---|---|

|
|
The results do not really open out the results particularly but give rise to some ideas that might explain the trends observed. The electron density on the electron rich carbon does infact decrease with increasing electronegativity of the elements (F=-0.812,Cl=-0.892, Br=-0.964) which does help explain why the Br acid is going to be the worse acid. It doesn't explain however why the fluorine superacid is the worse acid. In order to determine the true reason for the difference in acidity in the fluorinated superacid more advanced analysis is likely to be required. No analysis done reveals the reasons for this. Fluorine is a strange element with usually anomalous properties due to its high electronegativity. The reason for the difference is going to be down to some orbital effect but this has not been identified in this case. It again worth noting the effect is likely to be subtle given that firstly there is error in the energy associated with the calculation and secondly the energy differnece is only small anyway to the chlorinated species compared to that of the fully brominated case which is way up at 50.2934 kJmol-1.
Another thing that is interesting to look at is the calculated 11B NMR for the calculated fully chlorinated superacid. Here is the spectrum:
Boron NMR of Fully Chlorinated superacid (Unable to publish to D-space)
The important thing to take from this NMR is the fact that the general paettern matches what one would expect in a 1:5:5 ratio. This is an important technique in classification of carborane type compounds. In 1967 11B NMR was used in order to prove the structure of the related carborane B11CH122- [5]. The fact that the peaks can be found in the 1:5:5 ratios proves that the C adopts an apical poistion. The apical boron directly opposite has the lowest chemical shift at -10.7871ppm(reference: B2H6 HF/6-31G(d) GIAO). The next closet five borons are at around -17.76ppm and then then five closest borons are found at around -20.41ppm. This means the closer to the carbon the more shielded appears to be on the borons. There will, i'm sure, be some aromatic type influence on the shifts also due to ring currents but this would require a lot furthur work to determine.
The next thing that is going to be looked at briefly out of interest is the reaction of carboryne with a alkyne in a Diels-Alder type reaction[6] :
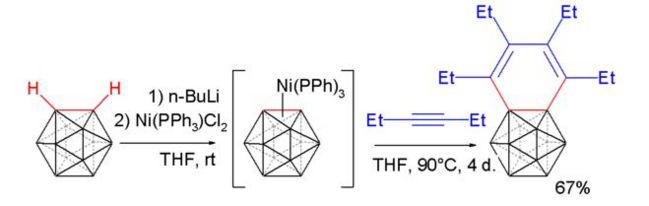
The interesting point about the product is that the potential benzene ring type structure (benzocarborane) actually shows no aromatic type structure over the benzene-type ring itself. The structure was optimised and an MO analysis run on it (pop=full) at the lanl2dz level.
# opt freq b3lyp/lanl2dz geom=connectivity pop=full
The bond lengths around the ring indeed suggest that the ring is not fully electron delocalised symmetrically (as in benzene's D6h symmetry type delocalisation) due to the variety of bond lengths around the system. These can be measured on the output to be 1.66, 1.50, 1.37 and 1.49 angstoms (going from the bnod included in the cluster to the furthest away single C-Cbond). These agree with the literature X-ray values fairly well [7] of 164.8 pm to 133.8 pm. This shows the calculation run is actually very accurate.
The structure optimised structure can seen below DOI:10042/to-1096 :
All of the MOs for this molecule where generated and examinated looking for orbitals on the benzene ring which would make it look aromatic in a symmetric way about the whole molecule. However these could not be found as expected. Given two of the carbons are encorporated into the carborane structure it is not expected that the benzene type delocalisation will be seen. i.e. boron based orbitals will interact with encorporated carbons breaking the symmetry of the "bezene-type" ring structure. There are however a few interesting orbitals which can be seen with analysis below:
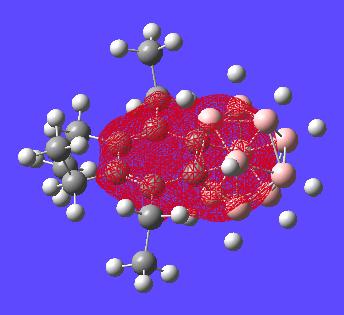
This MO shows both the carborane cluster and the "benzene" ring encorporated in the same orbital. this may suggect delocalisation of the electrons of the whole structure (two aromatic systems).
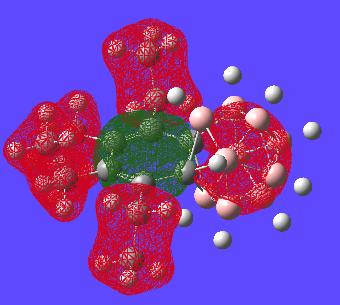
This orbital is the only orbital that looks the most like it has part of an orbital all in phase that only resides in a cyclic fashion on the benzene ring. This might suggest the bond lengths should really be alot closer in size to each other but clearly nearly all other MOs do not involve this type of delocalisation over the benzene and hence why the benzene ring has difference bond lengths.
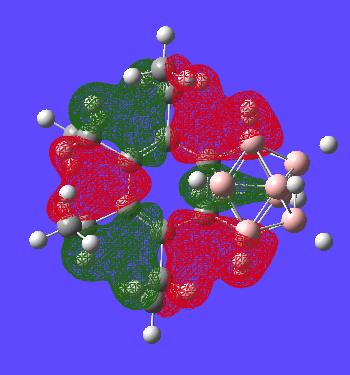
This MO is simply to show the that the benzene ring is generally symmetric as in normal benzene until the carborance cluster interferes with the orbitals as expected. The one (green) carborane based orbital is alot smaller than the other ones (again showing the lack of symmetry in the ring)
This part of the project really has no conclusion to draw other than that the bond lengths in the benzene ring match well to literature and that the benzene is not as aromatic or symmetric as normal benzene backed up roughly by MO visualisations of the molecule. This molecule could be researched in much more detail with other calculations however there is not time to do this in this project.
- ↑ 1.0 1.1 Inorganic Main Group-2nd Year lecture Course
- ↑ Aromaticity-Wikipedia-Aromaticity
- ↑ Hydrogen bond-WikipediaHydrogen Bond
- ↑ University of Waterloo-Bond lengths and bond energies-Website
- ↑ W. H. Knoth, Journal of the American Chemical Society, 1967, 89, 5, March 1, 1275 DOI:10.1021/ja00981a048
- ↑ Carboranes-Wikipedia Carborane
- ↑ Liang Deng, Hoi-Shan Chan, and Zuowei Xie (2006). "Nickel-Mediated Regioselective [2 + 2 + 2] Cycloaddition of Carboryne with Alkynes". J. Am. Chem. Soc. 128 (24): 7728–7729. DOI:10.1021/ja061605j

