Rep:Mod:ddaa3333
Module 1: Organic
Modelling using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
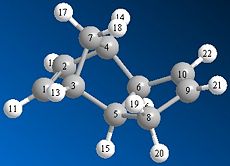
Cyclopentadiene can dimerise to form one of two products; the endo or exo dimer. Using a program such as ChemBio 3D the relative stability of each dimer can be determined and a decision as to which dimer is the kinetic and which dimer is the thermodynamic product can be made. The endo and exo dimers were drawn and their geometries optimised using the MM2 model. The results of this optimisation suggest that the exo dimer is the more thermodynamically stable product since it has a much lower total energy than the endo product; 31.89kcal/mol compared to 34.01kcal/mol respectively. The main reason for this difference in energy is due to the position of the hydrogen atoms at carbon 5 and 6 (see figure 1a. and 1b.) in relation to the bridgehead. In the exo dimer these two hydrogen atoms are orientated below the cyclopentadiene ring, on the opposite side to the bridgehead. Whereas in the endo dimer these two hydrogen atoms are orientated above the cyclopentadiene ring, on the same side as the bridgehead. This means that there is a much greater steric repulsion between these hydrogen atoms and the bridgehead in the endo dimer than in the exo dimer.
As can be seen in the table below the torsion energy of the endo dimer is much greater than the torsion energy of the exo dimer; 9.51kcal/mol compared to 7.63kcal/mol respectively.
We are told that the endo dimer is in fact the main dimer formed even though as mentioned above it has a much higher energy. This can be explained by considering the energy of the transition states that lead to the different dimers. The endo dimer has a lower energy transition state than the exo dimer and hence is the more kinetically stable product.
The endo dimer can undergo hydrogenation to give one of two products; dihydro derivative (3) or dihydro derivative (4) (see fig. 1e). The results of the optimisation of these two products suggest that derivative (4) is the more thermodynamically stable product since it has a much lower total energy than derivative (3); 31.16kcal/mol compared to 35.70kcal/mol respectively. As can be seen in the table below the bending energy of derivative (3) is much greater than the bending energy of derivative (4); 19.85kcal/mol compared to 14.49kcal/mol respectively.
 |
 |
 |
|
Stereochemistry of nucleophilic additions to a pyridinium ring (NAD+ analogue)
The nucleophilic addition of the grignard reagent MeMgI to the pyridinium ring (5) occurs via the mechanism shown in fig. 1f to give molecule (6).

This reaction has high regio and stereoselectivity since the methyl group of the MeMgI will only add to the pyridinium ring at the 4-position. The main reason for this high selectivity is that the Mg of the MeMgI coordinates to the amide oxygen [1]
Molecule (5) was drawn in ChemBio 3D and the MMFF94 force field option was used to determine its energy. Slight modifications were then made to the structure of this molecule to try to minimise its energy. Fig. 1g shows the lowest energy conformation of molecule (5). The energy of this conformation is 57.48kcal/mol.
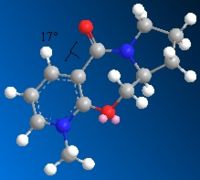
The nucleophilic addition of PhNH2 to the pyridinium ring (7) occurs via the mechanism shown in fig. 1g to give molecule (8).
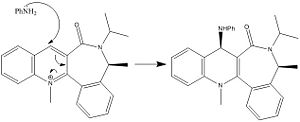
Just as for molecule (5), molecule (7) was drawn in ChemBio 3D and the MMFF94 force field option was used to determine its energy. Slight modifications were then made to the structure of this molecule to try to minimise its energy further. Figure. 1h shows the structure of (7) before it was altered. The energy of (7) as drawn in this figure was 98.45kcal/mol. Figures. 1i and 1j show the structure of (7) after modifications were made. In figure. 1i the orientation of the two isopropyl groups attached to the nitrogen atom was altered to see how this would affect the energy. The energy of (7) as drawn in this figure was 98.36kcal/mol. In figure. 1j the orientation of the amide oxygen was altered to see how this would affect the energy. The energy of (7) as drawn in this figure was 98.37kcal/mol.
Figures. 1i and 1j represent the lowest energy conformation of (7). In both figures the amide oxygen is orientated below the plane of the pyridinium ring. The reason why it is more favourable to have the oxygen orientated in this way is that it minimises the repulsion betwen the lone pair on the incoming nitrogen in PhNH2 with the lone pairs on this amide oxygen. This is the reason why for the stereochemistry of molecule (8).
 |
 |
 |
Stereochemistry and reactivity of an intermediate in the synthesis of Taxol
Figure. 1k shows the mechanism that leads to the formation of molecules (9) and (10). This is an example of a atropselective oxy-Cope rearrangement. [2] What this means is that rotation about certain single bonds in each molecule is severly hindered and hence the energy needed to induce rotation about these bonds is sufficiently high enough to allow for two separate isomers to be identified.
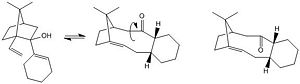
Molecules (9) and (10) were drawn using ChemBio 3D and their geometries optimised using the MM2 force field. The table to the left below shows the results of this optimisation. From these results it is clear to see that molecule (10) is more stable than molecule (9) since it has a much lower total energy; 48.20kcal/mol compared to 54.13kcal/mol respectively.
The geometries of molecules (9) and (10) were also optimised using the MMFF94 force field. The table to the right below shows the results of this optimisation. It should be noted that the energy of each molecule obtained using the MM2 and MMFF94 cannot be compared.
 |
 |
|
|
Molecules (9) and (10) both contain a bridgehead alkene which reacts much slower than expected for such a strained bond. These alkenes are hyperstable alkenes. This means that they have a negative strain energy[3] and so are not as destabilised by this strain as expected. Hyperstable alkenes were first reported by Bredt[4]
Modelling using semi empirical molecular orbital theory
Regioselective addition of dichlorocarbene
Molecular mechanic models were used to study the reactions above. In this section semi-empirical molecular mechanic models will be used. These models look at the effect electrons have on a reaction and how they influence certain physical and spectroscopic properties of a molecule.
The electrophilic addition of dichlorocarbene to molecule (12) can be studied using semi-empirical molecular orbital theory as can a hydrogenated version of molecule (12). Molecule (12) and its hydrogenated derivative were drawn in ChemBio 3D and their geometry optimised using the MM2 force field. The table below shows the results of these optimisations.
 |
 |
|
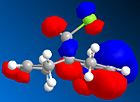
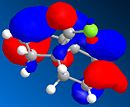
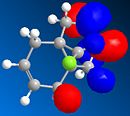
MOPAC/PM6 was then used to represent specific molecular orbitals of molecule (12); namely the HOMO, HOMO-1, LUMO, LUMO+1 and LUMO+2 (see below).
 |
 |
 |
 |
 |
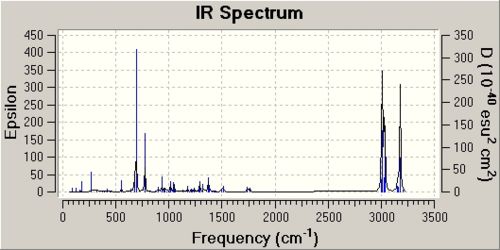
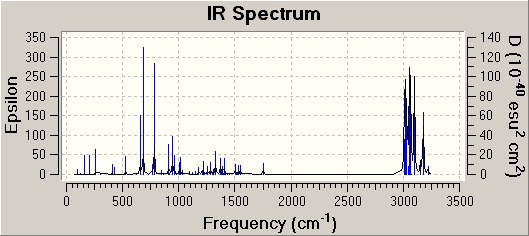
The antibonding Cl-C molecular orbital in molecule (12) (shown in LUMO+1 above) overlaps well with the bonding C=C orbital anti to the Cl-C bond (see HOMO-1 above). This effectively weakens the Cl-C bond and ultimately lowers its stretching frequency. When this C=C bond anti to the Cl-C bond is hydrogenated this orbital overlap is removed and so the Cl-C bond becomes stronger. This explains why the Cl-C stretching frequency in molecule (12) is less than the Cl-C stretching frequency in the hydrogenated derivative; 772.73cm-1 compared to 782.23cm-1 respectively. As discussed the Cl-C bond in the hydrogenated derivative is stronger than in the non-hydrogenated derivative; molecule (12). It is quite surprising therefore that the hydrogenate derivative has a longer bond length than the non-hydrogenated derivative; 1.751A compared to 1.747A respectively.
 |
 |
| ' | Frequency / (cm-1) | Infrared intensity |
| Cl-C stretch | 772.73 | 25.21 |
| Cl-C stretch | 931.86 | 7.65 |
| C=C Stretch (anti to Cl-C) | 1740.89 | 4.15 |
| C=C Stretch (syn to Cl-C) | 1761.02 | 3.9 |
| ' | Frequency / (cm-1) | Infrared intensity |
| Cl-C stretch | 782.23 | 22.34 |
| Cl-C stretch | 912.79 | 7.11 |
| Cl-C stretch | 948.86 | 9.12 |
| C=C Stretch (syn to Cl-C) | 1757.32 | 5.01 |
Mini project
Dynamic Kinetic Resolution of Atropisomeric Amides[5]
2-(1-Hydroxy-3-oxo-butyl)-naphthalene-1-carboxylic acid dicyclohexylamide- anti and syn isomers

Both the syn and anti isomers from the above reaction were drawn using ChemBio 3D and their geometries optimised using MM2 mechanics. The results of these optimisations are shown in figures. 1p and 1q below and the table to the right below. These optimisations suggest that the syn isomer is slighly more stable than the anti isomer since its total energy is just lower than that of the anti; 35.35kcal/mol compared to 36.48kcal/mol respectively.
 |
 |
|
DFT semi-empirical mechanic methods were then used to optimise the geometry of each isomer further using the basis set 6-31G. This optimisation changed the orientation of the O-H in the anti isomer so that it was planar to the aromatic rings.
 |
 |
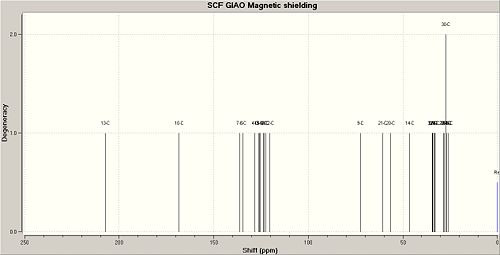 |
 |
| 13C{1H} Chemical Shift data (Anti isomer) / (ppm) | 13C{1H} Chemical Shift data Lit (Anti isomer) / (ppm) | Difference between literature values and calculated values / (ppm) | Correspnding to carbon number | 13C{1H} Chemical Shift data (Syn isomer) / (ppm) | 13C{1H} Chemical Shift data Lit (Syn isomer) / (ppm) | Difference between literature values and calculated values / (ppm) | Corresponding to carbon number |
| 26.2 | 25.4 | -0.8 | 29 | 26.0 | 25.7 | -0.3 | 29 |
| 26.4 | 25.7 | -0.7 | 24 | 26.6 | 25.8 | -0.9 | 24 |
| 27.4 | 25.8 | -1.6 | 30 | 27.3 | 25.9 | -1.4 | 25 and 30 |
| 27.5 | 25.9 | -1.6 | 28 | 28.0 | 27.0 | -1.0 | 28 |
| 27.7 | 27.0 | -0.7 | 25 | 28.4 | 27.1 | -1.3 | 23 |
| 28.2 | 27.1 | -1.1 | 23 | 32.9 | 30.1 | -2.8 | 22 |
| 32.6 | 30.1 | -2.5 | 22 | 33.1 | 30.3 | -2.8 | 26 |
| 33.1 | 30.5 | -2.6 | 27 | 34.1 | 31.4 | -2.7 | 27 |
| 34.2 | 30.9 | -3.3 | 31 | 34.3 | 31.4 | -2.9 | 17 |
| 34.3 | 31.5 | -2.8 | 17 | 34.4 | 31.5 | -2.9 | 31 |
| 34.7 | 31.7 | -3.0 | 26 | 46.5 | 49.3 | 2.8 | 14 |
| 52.2 | 52.0 | -0.2 | 14 | 56.6 | 57.1 | 0.5 | 20 |
| 53.6 | 56.7 | 3.1 | 20 | 60.7 | 61.0 | 0.3 | 21 |
| 59.7 | 60.5 | 0.8 | 21 | 72.5 | 68.1 | -4.4 | 9 |
| 76.2 | 67.9 | -8.3 | 9 | 120.4 | 123.5 | 3.1 | 12 |
| 121.0 | 123.5 | 2.5 | 6 | 122.7 | 125.4 | 2.7 | 6 |
| 122.6 | 125.4 | 2.8 | 12 | 123.3 | 126.8 | 3.5 | 2 |
| 123.1 | 126.6 | 3.5 | 2 | 123.6 | 127.2 | 3.6 | 1 |
| 123.8 | 127.1 | 3.3 | 1 | 125.6 | 128.5 | 2.9 | 5 |
| 125.7 | 128.5 | 2.8 | 11 and 3 | 125.7 | 129.2 | 3.5 | 3 |
| 126.9 | 129.0 | 2.1 | 5 | 126.4 | 129.7 | 3.3 | 11 |
| 127.2 | 129.5 | 2.3 | 4 | 128.2 | 133.2 | 5.0 | 4 |
| 130.3 | 133.2 | 2.9 | 8 | 134.5 | 135.0 | 0.5 | 8 |
| 137.6 | 135.2 | -2.4 | 7 | 136.3 | 136.1 | -0.2 | 7 |
| 169.2 | 169.4 | 0.2 | 10 | 168.4 | 170.5 | 2.1 | 10 |
| 205.5 | 210.8 | 5.3 | 13 | 207.4 | 207.4 | 0.0 | 13 |
The calculated 13C NMR for the syn and anti isomers can be found at DOI:10042/to-3705 and DOI:10042/to-3706 respectively. The NMR and IR data calculated using semi-empirical molecular mechanic methods gave similar results to the literature. For the NMR in particular the chemical shift values for each isomer only varied by a couple of ppm. This is likely to be due to changes in environments caused by the slight flexibility of each isomer. The NMR and IR data correctly identify each isomer.
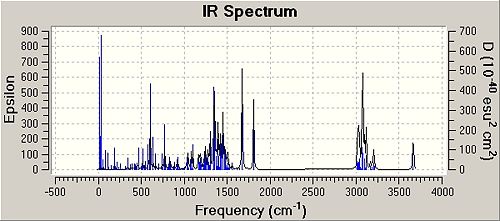 |
 |
| ' | Frequency Syn isomer / (cm-1) | Infrared intensity Syn isomer | Lit Frequency / (cm-1) | Frequency Anti isomer / (cm-1) | Infrared intensity Anti isomer | Lit Frequency / (cm-1) |
| Amide C=O stretch | 1673.98 | 157.66 | 1654.2 | 1673.68 | 148.75 | 1617.6 |
| Amide C=O stretch | 1675.79 | 89.17 | 1617.9 | 1675.43 | 117.53 | 1617.6 |
| O-H stretch | 3665.28 | 120.9 | 3413.2 | 3692.96 | 120.62 | 3446.7 |
| Carbonyl C=O stretch | 1808.08 | 131.48 | 1717.9 | 1812.6 | 179.88 | 1707.7 |
References
- ↑ Schultz. A, G. Flood. L, J. Org. Chem, 1986, 51, pp838-841
- ↑ Elmore, S. W, Paquette, L. A, Tetrahedron Letters, Vol.32, No.3,1991, pp319-322
- ↑ McEwen. A, B and Rague Schleyer. P. J. Am. Chem. Soc. 1986, 108, pp3951-3960
- ↑ Bredt, J. Thoret, H. and Schmitz, J. Liebigs Ann. Chem. 1924, 437, pp1
- ↑ Chan, V. Gon Kim, J. Jimeno, C. Carroll, P. J. and Walsh, P. J. Organic Letters, 2004, vol 6, No 12, pp2051-2053