Rep:Mod:dcn2842
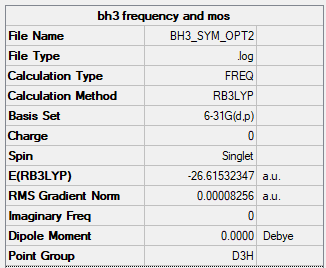
BH3
| Name | BH3 |
|---|---|
| Calculation Method | B3LYP |
| Basis Set | 6-31G(d,p) |
| Final Energy (au) | -26.6153 |
| RMS Gradient (au) | 0.00008256 |
| Point Group | D3H |
Item Value Threshold Converged? Maximum Force 0.000165 0.000450 YES RMS Force 0.000083 0.000300 YES Maximum Displacement 0.000650 0.001800 YES RMS Displacement 0.000325 0.001200 YES
Low frequencies --- -0.2407 -0.1105 -0.0054 44.9600 46.0933 46.0939 Low frequencies --- 1163.6314 1213.6102 1213.6129
Optimised BH Molecule |
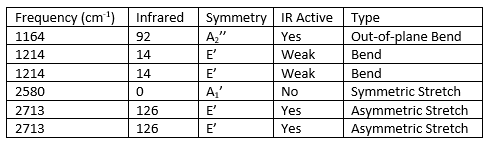
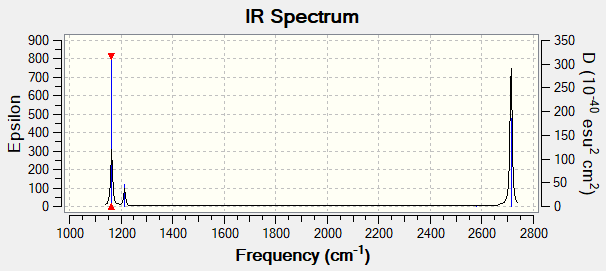
Why are there less than six peaks in the spectrum, when there are obviously six vibrations.
Although the table lists six frequencies where peaks should be located, only three are visible in the spectrum. This is as the peaks at 1214cm-1 and 2713cm-1 are degenerate and therefore overlapping, thus only showing one peak for each. Also, the vibration at 2580cm-1 is not IR active as there is no change in dipole with the vibration.
Molecular Orbitals of BH3
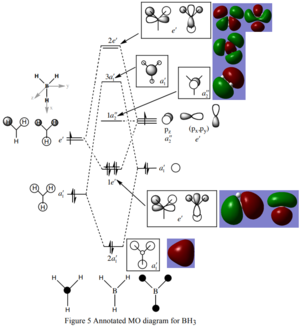
Are there any significant differences between the real and LCAO MOs?
LCAO MOs show each individual AO of the atom, while real MOs have an overlap of MOs of the same phase and cancelling out of opposite phase MOs, showing interaciton between the MOs.
What does this say about the accuracy and usefulness of qualitative MO theory?
Qualitative MOs give a rough estimate of the shape and position of real MOs, which provide information to approximately visualise real MOs.
Ng611 (talk) 20:24, 20 May 2019 (BST) Can you see any discrepancies?
Association Energies
E(NH3)=-56.5578au
E(BH3)=-26.6153au
E(NH3BH3)=-83.2247au
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
ΔE=-0.0516au
ΔE=-135.47581kJ/mol
Bond energy = 135kJ/mol
This is a sensible approximation for the B-N dative bond due to its magnitude. Comparing with bond energies of other boron bonds such as a B-H bond, which is 389kJ/mol[2], the B-N dative bond is a relatively weak bond.
Ng611 (talk) 20:25, 20 May 2019 (BST) Good calculation, good comparison.
NI3
Item Value Threshold Converged?
Maximum Force 0.000061 0.000450 YES
RMS Force 0.000037 0.000300 YES
Maximum Displacement 0.000459 0.001800 YES
RMS Displacement 0.000285 0.001200 YES
Low frequencies --- -5.5593 -5.4903 -0.0003 -0.0001 0.0000 6.5127 Low frequencies --- 101.1566 101.2827 148.4563
Optimised NI3 |
The optimised bond length calculated was to be 2.184Å.
Day 2&3: Lewis Acid and Bases
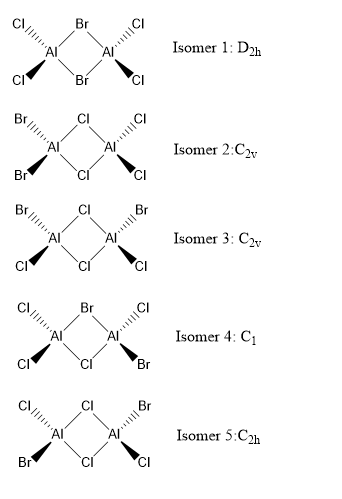
Isomers of Al2Cl4Br2
Summary Pages
Ng611 (talk) 20:28, 20 May 2019 (BST) You've used the wrong basis set here (6-31++G**/LANL2DZ should have been used here) and have obtained incorrect energies as a result. We also needed the remaining information (freq file, jmol, convergence table etc.) for the relevant calculations.
Compute the energy of the isomers with (a) 2 bridging Br ions and (b) the isomer with trans terminal Br and bridging Cl ions
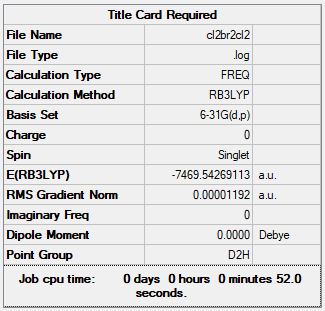
Isomer with 2 briding Br ions: Isomer 1
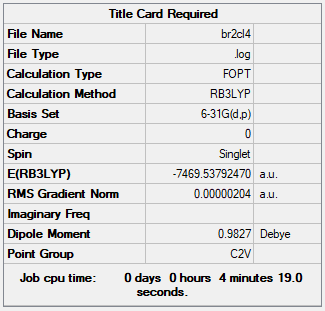
Isomer with trans terminal Br and briding Cl ions: Isomer 5
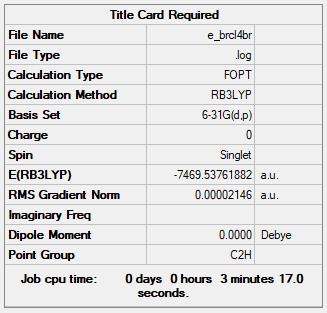
E of isomer 1 = -7469.54269au = 19611285.827kJ/mol
E of isomer 5 = -7469.53762au = 19611272.515kJ/mol
Isomer 1 (bridging with Br ions) is (19611285.827 - 19611272.515 = ) 13kJ/mol more negative than isomer 5. This indicates that Al2Cl4Br2 with Br bridging ions and D2h symmetry is more stable than having Cl bridging ions.
Ng611 (talk) 20:31, 20 May 2019 (BST) You've got it the wrong way around here.
Determine the dissociation energy for the lowest energy conformer into 2AlCl2Br
The lowest energy conformer is isomer 1, where E of isomer 1 = -7469.54269au. To calculate the dissociation energy to AlCl2Br, the energy of AlCl2Br was optimised for and was calculated to be:
E(AlCl2Br) = -3734.74852au[3]
Using this information, the dissociation energy was calculated to be:
-7469.54269 - 2(-3734.74852) = -0.04565 au = -120 kJ/mol
This therefore indicates that the product is more stable than the isolated monomers of AlCl2Br as the isomer has energies
Ng611 (talk) 20:31, 20 May 2019 (BST) Correct trend but wrong number ultimately. Where's you calculation for AlCl2Br
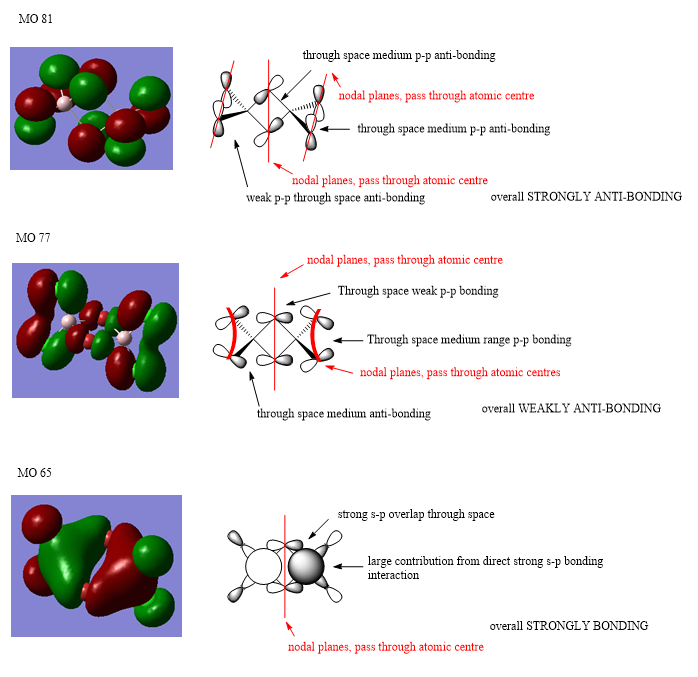
Molecular Orbitals of the Most Stable Isomer (Isomer 1)
Ng611 (talk) 20:34, 20 May 2019 (BST) Excellent MO analysis. You did well in your first section but let yourself down in the second...
References
- ↑ Patricia Hunt, Lecture 4 Tutorial Problem Model Answer, Imperial College London, 9th May
- ↑ Huheey, pps. A-21 to A-34; T.L. Cottrell, "The Strengths of Chemical Bonds," 2nd ed., Butterworths, London, 1958; B. deB. Darwent, "National Standard Reference Data Series," National Bureau of Standards, No. 31, Washington, DC, 1970; S.W. Benson, J. Chem. Educ., 42, 502 (1965)
- ↑ File:Mh4417ALCL2BR.LOG