Rep:Mod:db9127organic
The Hydrogenation of Cyclopentadiene Dimer
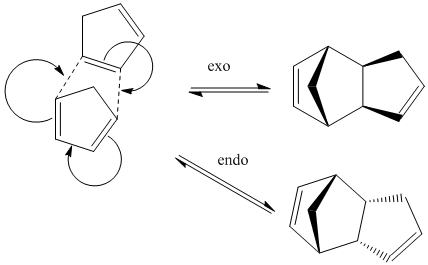
Cyclopentadiene dimerises to form specifically the endo product shown below.
Product_1 |
Exo
Product_2 |
Endo
The exo product has a lower energy (31.9kcal/mol as opposed to 34.0kcal/mol.) This means that the exo product is thermodynamically more stable than the endo product. However, the endo product (as with most Diels- Alder cycloaddition reactions) predominates. This means that the reaction is kinetically controlled, with the endo product having a transition state with a lower energy than that of the exo product. This stereospecificity is due to secondary orbital overlaps, that stabilise the transition state.
The endo product can be hydrolysed to form one of the two products shown:
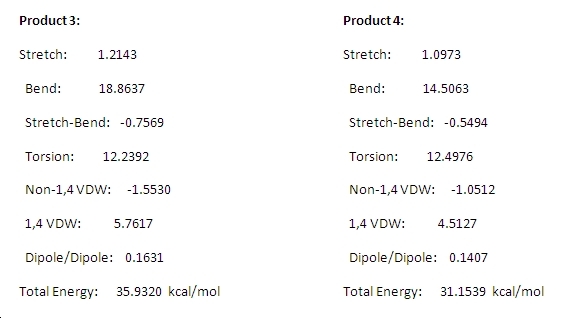
Product_3 |
Product 3
Product_4 |
Product 4
The energies of the two products are shown here:
The majority of the difference between the total energies comes from the greater stretching and bending energies of product 3. However, product 3 also has greater energy contributions in every respect apart from torsion. The difference in torsion energies is probably due to the double bond in product 3 being part of a more rigid pentane ring structure. The difference in total energies makes 4 thermodynamically more stable. This implies that the double bond that remains present in 3 is more easily hydrolysed under thermodynamic conditions (equilibrium). The kinetic product is unknown as the energies of transition states are unknown and it is not known which of the products predominates the reaction under kinetic control.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
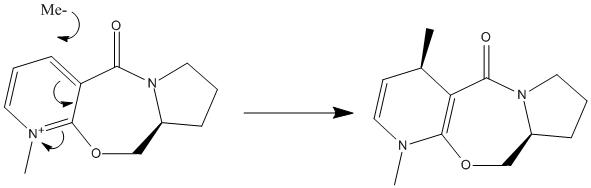
Methylation of Prolinol
Prolinol can be methylated at the 4 position using a Grignard reagent such as MeMgI. It creates a stereospecific isomer as shown above. The stereospecificity is due to the conjugation of the methyl carbanion to the carbonyl oxygen. This reduces the energy of the transition state when the nucleophile attacks from the correct geometric alignment.[1] This forms the kinetic product. The alternative isomer is actually thermodynamically more stable (lower energy) but requires a much greater activation energy during formation so it is not formed. The reactant and product are shown here with the dihedral angle of the carbonyl oxygen atom to the pyridine ring, to show that the methyl group is attached cis to the carbonyl group:
Prolinol
Pprolinol |
Dihedral angle = 24 degrees.
4-Methylprolinol
Pentahelicene |
Dihedral angle = 11 degrees.
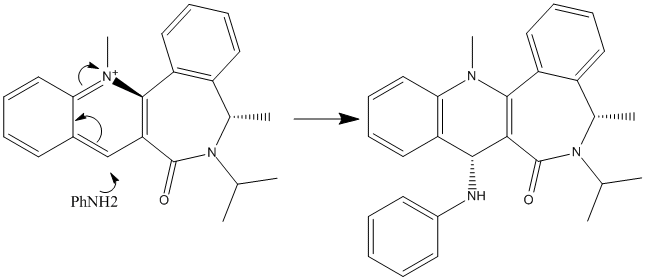
Nitrophenylation of a pyridine derivative
This reaction is stereospecific in the same way as the reaction above. Again, the carbonyl group is in a fixed conformation relative to the pyridine ring, making the nucleophile attack cis to the carbonyl oxygen atom. Effectively the nucleophile bonds to the oxygen atom first and then migrates onto the 4 position on the pyridine ring. This greatly reduces the activation energy of the reaction and this makes this isomer completely dominate the reaction.[1] The reactant and product are shown here, with the same dihedral angles calculated:
Reactant (7)
Pprolinol |
Dihedral angle = 42 degrees.
Product (8)
Pprolinol |
Dihedral angle = 51 degrees.
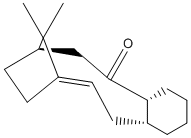
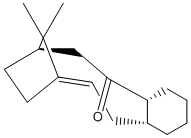
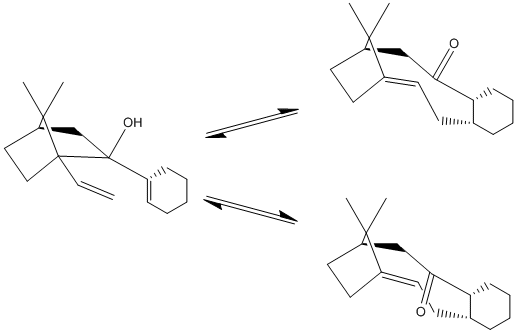
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
During the synthesis of taxol this intermediate is formed with the 2 posssible conformations shown:
This molecule isomerises to form the isomer 11, as this is far more thermodynamically stable. This is done via the oxy-cope rearrangement shown here:
Isomer 10 has an energy of 127.9kcal/mol whereas isomer 11 has an energy of 48.2kcal/mol. This makes 11 far more thermodynamically stable and therefore the dominant product. However, this orientation of the carbonyl group does not enable the functionalisation of the alkene group, as it is orientated away from the alkene group and any group bonding to the carbonyl oxygen atom cannot then react intramolecularly with the double-bond. This makes the functionalisation of the alkene very slow, as it has a high activation energy.
How one might induce room temperature hydrolysis of a peptide
This reaction shows the hydrolysis of a peptide bond under standard conditions and how the stereochemistry of the reactant changes the rate of reaction. This particular hydrolysis is much faster than a typical peptide bond hydrolysis because it is an intramolecular reaction, which greatly increases the chance of a reaction-causing collision occurring. The two isomers shown react at very different speeds due to the conformation of the ring. With isomer 13, the ring is folded as shown in the 3D structure, bringing the -OH group much closer to the carbonyl group of the peptide bond (which it must collide with to react) than in isomer 14.
There are two stereoisomers of each of 13 and 14, with the -CH2CONH2 group either axial or equatorial to the ring. However, these structures can interconvert due to the presence of a lone pair of electrons on the nitrogen atom. Hence, these structures only neeed to be identified for modelling purposes. The reacting structure is the equatorial structure for 13 and the axial for 14, as this allows it to effectively swing towards the -OH group with which it must react. All four isomeric structures are shown below, with the reacting ones marked.
Peptide13 |
13
Peptide13b |
13 (reactant conformer)
Peptide14 |
14 (reactant conformer)
Peptide14b |
14
For isomer 13, hydrogen bonds can form between the hydroxyl group and the peptide nitrogen when in the equatorial conformation. This stabilises this conformation, making it far more energetically stable than the axial conformation. (the equatorial conformation is more stable anyway, due to steric effects.) This increases the rate of reaction for isomer 13, as it brings the hydroxyl group and the carbonyl group closer together. Conversely, isomer 14 must interconvert to the axial conformation to react and there is a hydrogen bond between the hydroxyl group and the ring-bound nitrogen atom in the equatorial conformation.[2] This increases the energy of interconversion, therby increasing the activation energy and decreasing the rate of reaction. The energy of interconversion for 14 is 7.96kcal/mol. (found by the difference between conformational energies)
Regioselective Addition of Dichlorocarbene
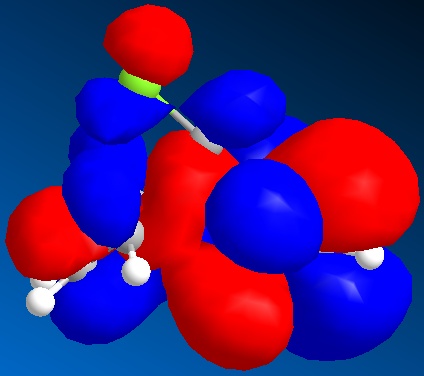
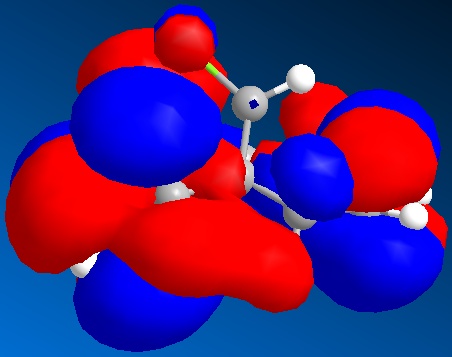
Calculating molecular orbitals for a structure can be used to determine regioselectivity. For the diene shown below, the HOMO orbital was mapped to demonstrate which double-bond was more susceptible to electrophilic attack.
The HOMO shows a far greater electron density on the double bond that is endo to the chlorine atom. This is due to donation from a lone pair of electrons on the chlorine atom. This increase in electron density makes this double bond more susceptible to electrophilic attack, and demostrates the regioselectivity of the molecule. This is because the HOMO will be the orbital that takes part in the reaction, as it donates electron density to an electrophile.
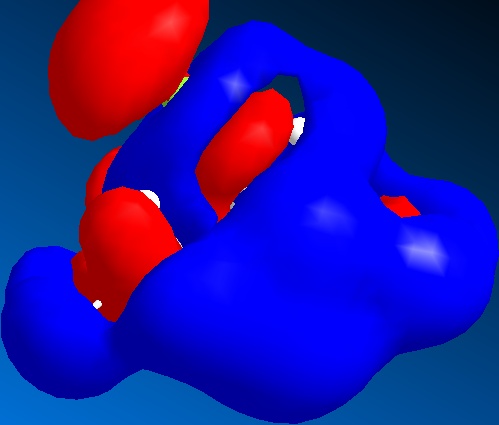
The other nearby orbitals will also determine the orientation of the incoming electrophile. This is called secondary orbital contribution. For this reason, the HOMO-1, LUMO, LUMO+1 and LUMO+2 are shown. These also favour the endo as a site for attack, with a decreased electron density on the endo double bond amongst the LUMO, which would accept electron density from an incoming reactant.
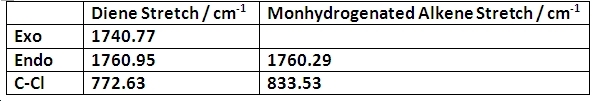
The following similar molecule ( a monohydrogenated version of the diene) was then optimised for comparison of bond energies. This was done by calculating the IR stretching vibrations of the C-Cl bond and the alkene double bonds. These are shown below:
Vibrational frequency is directly proportional to bond strength. Hence, it can be concluded that the presence of an exo double bond does little to affect the bond strength of the endo double bond but greatly increases the bond strength of the C-Cl bond. This is because this exo alkene donates electron density onto the chlorine atom.
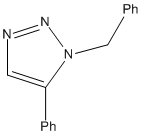
Assigning regioisomers in "Click Chemistry"
The 1,3-dipolar cycloaddition reaction shown below is an example of a "click" reaction. These reactions are so fast with the correct catalysts that they are referred to as "click" reactions. This particular reaction can form two stereospecific products depending on the catalyst used.
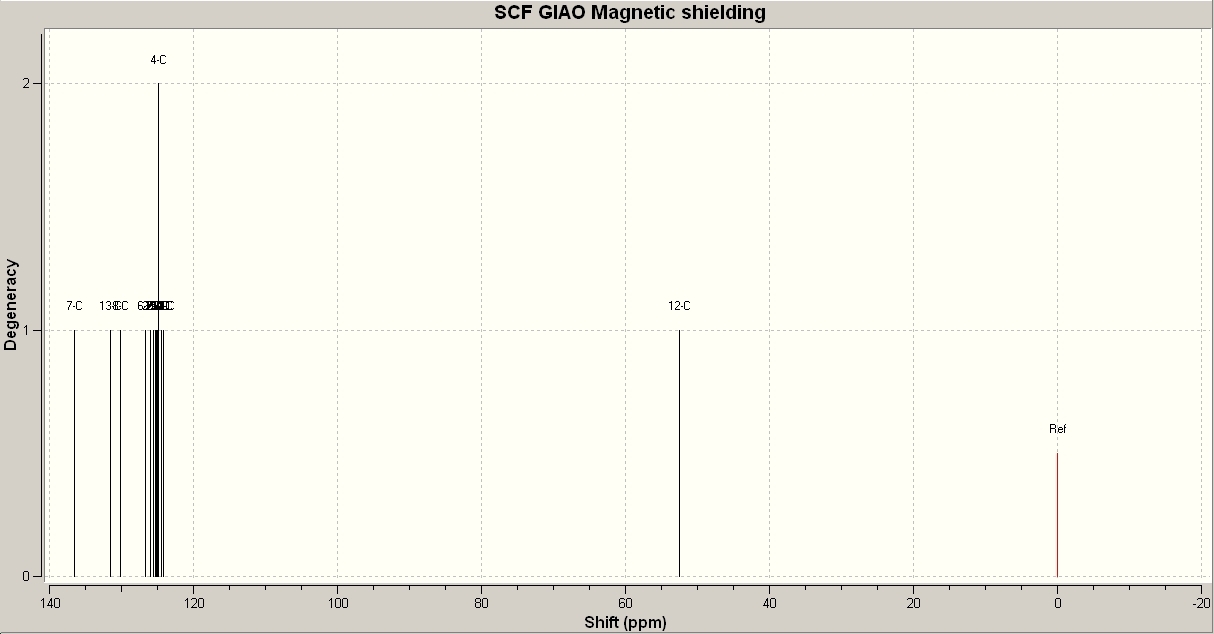
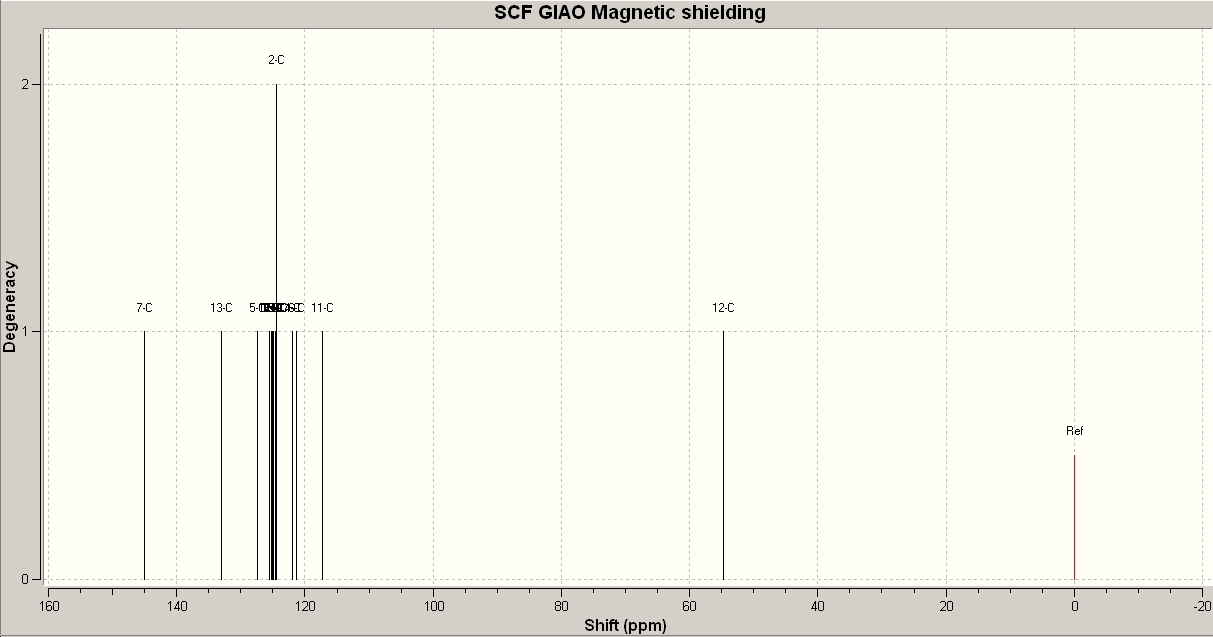
Product A is produced exclusively when a Cu(I) catalyst is used. However, product B predominates in the presence of a Ru(II) catalyst. These two products can be identified by using spectroscopy that targets the environment of the carbon atoms in the azide ring. For example, 13C NMR spectroscopy will determine which product is found by the difference in shift between that particular carbon atom in each product. 1H NMR spectroscopy could determine the environment of the proton attached to this carbon atom, but this would be a less clear change. IR Spectroscopy could detect a difference in the nitrogen bond stretches due to the proximity of the phenyl groups but this would be a minimal change and difficult to accurately detect. 13C NMR spectra were calculated using Gaussian mechanics to demonstrate the difference in the environments of the azide ring carbon atoms. The spectra are shown below:
As can be seen, there are only two major differences between the two spectra. Product A has a peak at 136.6ppm. This refers to the carbon atom in the azide ring with a phenyl group attached. Product A also has a peak at 130.1ppm which refers to the other carbon atom in the azide ring. Product B has a peak at 145.0ppm which refers to the phenylic carbon in the azide ring and a peak at 117.3 ppm which refers to the other azide carbon atom. According to the literature values, the first peak for A should be at 138.3ppm. This shows some of the inaccuracy in calculating NMR spectra in this way, but the difference between A and B is considerably greater than the difference between calculated and literature values, which shows that this is a reliable way to distinguish between products.
A mechanism has been suggested for the regiospecificity of the Ru(II) catalysts. This is shown here, with the assumption that intermediate A is formed more readily than B:
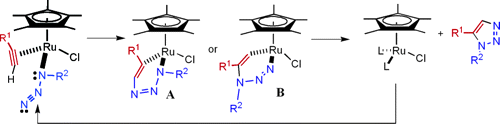 [3]
[3]
- ↑ 1.0 1.1 A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ M. Fernandes, F. Fache, M. Rosen, P.-L. Nguyen, and D. E. Hansen, 'Rapid Cleavage of Unactivated, Unstrained Amide Bonds at Neutral pH', J. Org. Chem., 2008, 73, 6413–6416 ASAP DOI:10.1021/jo800706y
- ↑ Picture taken from J. Am. Chem. Soc. 2005, 127, 15998; DOI:10.1021/ja054114s