Rep:Mod:daretoeatapeach
MODULE 3 Anshumalita Patel
Cope Rearrangement
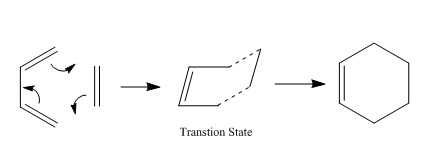
The Cope Rearrangement is a [3,3]-sigmatropic rearrangement involving 1,5 dienes. It is concerted and pericyclic but can also be thought of as going through a transition state which is similar in structure and energy to a diradical.[1] In order to study chemical reactivity, the Cope Rearrangement of 1,5 hexadiene will be analysed and the low-energy minima and transition structures on the C6H10 potential energy surface will be found so that the preferred reaction mechanism can be determined.
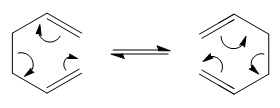
Optimisation of 1,5-Hexadiene Conformers
Firstly the optimised structures for the reactants and the products need to be found. The three middle sigma bonds can rotate, making numerous conformers possible for this molecule. However the lowest energy conformer must be found (as this will be the preferred transition and this can be calculated using Gaussview and the Hartree-Fock method with the 6-31G (d) basis set. The two low energy conformations (gauche and anti-periplanar) are drawn in Gaussview and optimised so that their respective energies can be determined. What would be expected is that the anti conformation would be lower in energy due to the reduced steric hindrance of the two groups pointing in opposite directions.
| Conformer | Optimised Structure | Energy (Atomic Units) | Point group |
| Anti-periplanar |  |
-231.69254 | Ci |
| Gauche |  |
-231.69153 | C2 |
In the anti-periplanar conformation above the olefin groups are still parallel to one another and so the energy might be lowered if they were adjusted so that they were trans to one another.
The DFT-B3LYP 6-31G* calculation gave the anti conformer as -234.61171 a.u and Ci point group
Frequency Analysis of 1,5 Hexadiene Conformers
Optimising the Chair and Boat Transition States
Optimisation of the Chair Transition State
Optimisation of the Boat Transition State
Intrinsic Reaction Coordinate Method for the Chair Transition State
Diels Alder Cycloaddition of Cis-Butadiene and Ethylene


Diels Alder Cycloaddition of Maleic Anhydride and Cyclohexa-1,3-diene
References
- ↑ Arthur C. Cope; et al.; J. Am. Chem. Soc. 1940, 62, 441.