Rep:Mod:cpyx
Introduction
The Gaussian software is a very useful tool in computational chemistry. In the computational physical chemistry lab course, this software was used to optimise the structures of molecules of ammonia, nitrogen, hydrogen as well as the ammonium ion. The bond lengths, bond angles, point groups, final energies and RMS gradient norms of the optimised structures of these species were found. The energies of ammonia, nitrogen and hydrogen were used to determine the reaction energy of the Haber-Bosch process, where ammonia is formed from nitrogen and hydrogen. The vibrations and molecular orbitals of all these species were also analysed using the GaussView software. The atomic charges of ammonia and the ammonium ion were also analysed and compared.
NH3 molecule
General information
| NH3 | ||||
|---|---|---|---|---|
| Calculation | OPTF | |||
| Method | B3LYP | |||
| Basis Set | 6-31G(d,p) | |||
| RMS gradient norm (a.u.) | 0.00000485 | |||
| Final energy E (a.u.) | -56.55776873 | |||
| Point group | C3v | |||
| N-H bond length (angstroms) | 1.01798 | |||
| H-N-H bond angle (degrees) | 105.741 | |||
Optimisation table
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000004 | 0.000450 | YES |
| RMS Force | 0.000004 | 0.000300 | YES |
| Maximum Displacement | 0.000072 | 0.001800 | YES |
| RMS Displacement | 0.000035 | 0.001200 | YES |
Predicted change in Energy= -5.986294D-10
The optimisation file is linked to here
Vibrational modes
NH3 is a non-linear molecule with 4 atoms. Using the 3N-6 rule, I expect to see 3(4)-6 = 6 vibrational modes. The frequencies of these 6 vibrational modes are shown below.
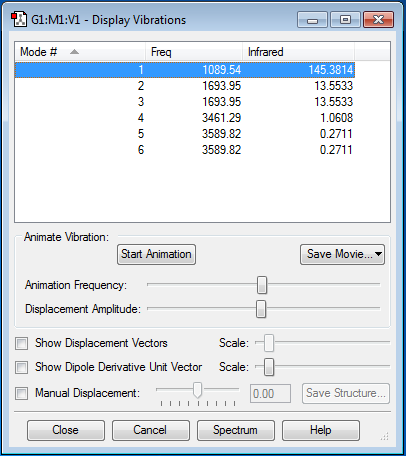
As shown in the table above, some bending and stretching modes have the same frequency, meaning that they have the same energy and are therefore degenerate. Modes 2 and 3 are degenerate, as well as modes 5 and 6.
Modes 1, 2 and 3 are "bending" vibrations whereas modes 4, 5 and 6 are "bond stretch" vibrations. It can be seen from the table above that "bending" vibrations have much lower frequencies as compared to "bond stretch" vibrations.
Mode 4, which is the symmetric stretching of all the N-H bonds, is a highly symmetric mode. Mode 1 is the bending of all the N-H bonds in a similar direction, such that all the bonds bend inwards together or all the bonds bend outwards together. It is known as the "umbrella" mode as the bending is very similar to the way that an umbrella is opened up and closed. As all the N-H bonds bend in a similar direction in mode 1, there is a large change in dipole moment of the molecule, resulting in a high IR intensity of 145.3814.
Although there are 4 distinct vibrational modes, I would only expect to see 2 bands in an experimental IR spectrum of gaseous ammonia. These correspond to mode 1 and degenerate modes 2 and 3. As shown in the right column in the table above, the IR intensities of modes 4, 5 and 6, which are all "bond stretch" vibrations, are very low as compared to the IR intensities of modes 1,2 and 3, which are all "bending" vibrations. The reason for the low IR intensities of the "bond stretch" vibrations as compared to the "bending" vibrations in NH3 is that "bond stretch" vibrations cause a very small change in dipole moment of this particular molecule, much smaller than the change in dipole moment caused by "bending" vibrations. Therefore, the intensities of the peaks in the IR spectrum corresponding to modes 4,5 and 6 are too low to be seen.
Atomic charges
| Atom | Charge |
|---|---|
| Nitrogen | -1.125 |
| Hydrogen | +0.375 |
NH3 is a neutral molecule with covalent bonding between the central nitrogen atom and the hydrogen atoms. As nitrogen is a more electronegative element as compared to hydrogen, the shared electrons in each of the 3 N-H bonds in the molecule will be pulled closer to the central nitrogen atom. These N-H bonds are polar covalent bonds. Thus, I expect the nitrogen atom to take on a negative charge and the 3 hydrogen atoms to all take on the same positive charge, which is really the case as shown in the table above. Adding up all the charges of the nitrogen atom and the 3 hydrogen atoms shown above gives 0, which shows that NH3 is indeed a neutral molecule.
N2 molecule
General information
| N2 | ||||
|---|---|---|---|---|
| Calculation | OPTF | |||
| Method | B3LYP | |||
| Basis Set | 6-31G(d,p) | |||
| RMS gradient norm (a.u.) | 0.00000060 | |||
| Final energy E (a.u.) | -109.52412868 | |||
| Point group | D∞h | |||
| N-N bond length (angstroms) | 1.10550 | |||
Optimisation table
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000001 | 0.000450 | YES |
| RMS Force | 0.000001 | 0.000300 | YES |
| Maximum Displacement | 0.000000 | 0.001800 | YES |
| RMS Displacement | 0.000000 | 0.001200 | YES |
Predicted change in Energy= -3.401087D-13
The optimisation file is linked to here
Display vibrations
| Frequency | Infrared |
|---|---|
| 2457.33 | 0.0000 |
Only 1 vibrational mode is shown, which is the stretching of the N-N triple bond. As a molecule of N2 contains only 2 identical nitrogen atoms, it does not have a dipole moment. Therefore, this "bond stretch" vibration does not cause a change in dipole moment and is not detected in IR spectroscopy, resulting in an IR intensity of 0.
Molecular orbitals
When 2 atoms form a molecule, the molecular orbitals (MO) are constructed from atomic orbitals by linear combination. The total number of electrons in the MO diagram is the total number of electrons in the whole molecule. A nitrogen atom contains 7 electrons and has an electronic configuration of 1s22s22p3, so the diatomic N2 molecule has a total of 14 electrons. The covalent bond between the 2 nitrogen atoms in this molecule is a triple bond, so all the 3 electrons in the 2p orbitals in each nitrogen atom are involved in bonding. The 2p electrons form 1 sigma and 2 pi bonds. There is stabilisation in this bonding as the corresponding antibonding orbitals of these bonding orbitals are empty and do not contain electrons. Bonding orbitals have a lower energy while antibonding orbitals have a higher energy than the corresponding atomic orbitals.
Although all the 14 electrons in the N2 molecule are involved in bonding, the stabilisation achieved by the filling of the 1s and 2s sigma bonding orbitals is cancelled out by the destabilisation caused by the filling of the corresponding antibonding orbitals. This means that the stabilisation of the N2 molecule as compared to the individual nitrogen atoms is due to bonding between the electrons in all the 2p orbitals in the molecule.
When a molecule donates electrons to another substance, the electrons from the highest occupied molecular orbital (HOMO) are removed so as to remove the most energy from the molecule. Electrons that are donated to the molecule will be placed in the unoccupied molecular orbital with the lowest energy level, known as the lowest unoccupied molecular orbital (LUMO). The table below shows representations of the HOMO and LUMO of N2.
| HOMO | LUMO |
|---|---|
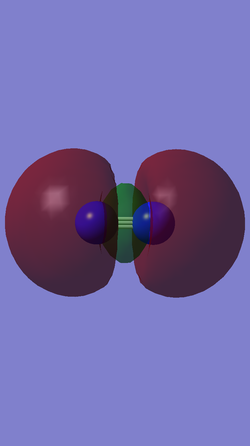 |
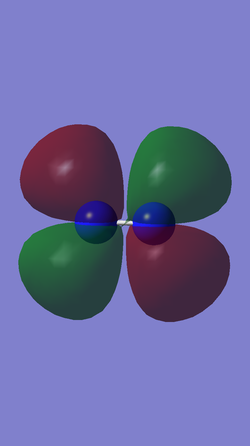
|
H2 molecule
General information
| H2 | ||||
|---|---|---|---|---|
| Calculation | OPTF | |||
| Method | B3LYP | |||
| Basis Set | 6-31G(d,p) | |||
| RMS gradient norm (a.u.) | 0.00005270 | |||
| Final energy E (a.u.) | -1.17853935 | |||
| Point group | D∞h | |||
| H-H bond length (angstroms) | 0.74292 | |||
Optimisation table
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000091 | 0.000450 | YES |
| RMS Force | 0.000091 | 0.000300 | YES |
| Maximum Displacement | 0.000120 | 0.001800 | YES |
| RMS Displacement | 0.000170 | 0.001200 | YES |
Predicted change in Energy= -1.063105D-08
The optimisation file is linked to here
Display vibrations
| Frequency | Infrared |
|---|---|
| 4463.86 | 0.0000 |
Like N2, there is only 1 vibrational mode in H2. In this case, the vibrational mode is the stretching of the H-H bond. As a molecule of H2 contains only 2 identical hydrogen atoms, it does not have a dipole moment. Therefore, this "bond stretch" vibration does not cause a change in dipole moment and is not detected in IR spectroscopy, resulting in an IR intensity of 0.
The frequency of the "bond stretch" vibration in H2 is much higher than the frequency of the "bond stretch" vibration in N2 as the reduced mass of H2 is smaller than the reduced mass of N2. Reduced mass is inversely proportional to vibrational frequency.
Molecular orbitals
The table below shows representations of the HOMO and LUMO for the H2 molecule. An atom of hydrogen contains 1 electron and hydrogen has an electronic configuration of 1s1. Thus, there are only 2 electrons in a molecule of H2, and both electrons are involved in sigma bond formation between the 2 hydrogen atoms in the molecule. The HOMO is the sigma bonding orbital and the LUMO is the sigma* antibonding orbital.
| HOMO | LUMO |
|---|---|
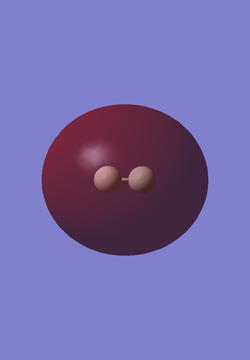 |
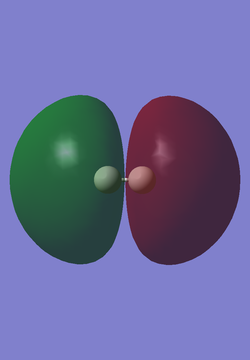
|
The Haber-Bosch process
The chemical equation for this process is:
N2 + 3H2 2NH3
All data recorded in the table below is in au.
| E(NH3) | -56.55776873 |
| 2*E(NH3) | -113.11553746 |
| E(N2) | -109.52412868 |
| E(H2) | -1.17853935 |
| 3*E(H2) | -3.53561805 |
| ΔE=2*E(NH3)-[E(N2)+3*E(H2)] | -0.05579073 |
ΔE = -0.05579073 x 2625.5 = -146.478561615 kJ/mol
ΔE is negative, indicating that energy is released from the system to the surroundings when ammonia is formed from the gaseous reactants of nitrogen and hydrogen. This means that the ammonia product is lower in energy and more thermodynamically stable than the reactants. The forward reaction producing ammonia is favoured.
NH4+ ion
General information
| NH4+ | ||||
|---|---|---|---|---|
| Calculation | OPTF | |||
| Method | B3LYP | |||
| Basis Set | 6-31G(d,p) | |||
| RMS gradient norm (a.u.) | 0.00011991 | |||
| Final energy E (a.u.) | -56.90586436 | |||
| Point group | Td | |||
| N-H bond length (angstroms) | 1.02761 | |||
| H-N-H bond angle (degrees) | 109.471 | |||
Comparison of H-N-H bond angle between NH3 and NH4+
The difference between NH4+ and NH3 is that NH4+ contains 1 more proton. NH3 and NH4+ are conjugate acid-base pairs, with NH4+ being the conjugate acid and NH3 being the conjugate base.
Both NH3 and NH4+ have tetrahedral pseudostructures. NH4+ contains 4 bond pairs and no lone pairs of electrons around the central nitrogen atom. On the other hand, NH3 contains 3 bond pairs and 1 lone pair of electrons around the central nitrogen atom. Thus, NH4+ has a tetrahedral structure whereas NH3 has a trigonal pyramidal structure.
According to the Valence Shell Electron Pair Repulsion (VSEPR) theory, lone pair-lone pair repulsions are considered to be stronger than lone pair-bond pair repulsions and lone pair-bond-pair repulsions are considered to be stronger than bond pair-bond pair repulsions. There are no lone pair-lone pair repulsions within a NH3 molecule and within a NH4+ ion. However, there are lone pair-bond pair repulsions within a molecule of NH3 due to the lone pair of electrons on the central nitrogen atom and the 3 electron pairs in the 3 N-H bonds. NH4+ does not have any lone pair of electrons on the central nitrogen atom, so there are no lone pair-bond pair repulsions exhibited in NH4+. This results in the H-N-H bond angle in NH3 being smaller than the H-N-H bond angle in NH4+.
The H-N-H bond angles found by optimisation of the structures of NH3 and NH4+ are 105.741 degrees and 109.471 degrees respectively. Clearly, the H-N-H bond angle in NH3 is found to be smaller than the H-N-H bond angle in NH4+. Therefore, these bond angles found are consistent with the VSEPR theory.
Optimisation table
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000232 | 0.000450 | YES |
| RMS Force | 0.000124 | 0.000300 | YES |
| Maximum Displacement | 0.000538 | 0.001800 | YES |
| RMS Displacement | 0.000288 | 0.001200 | YES |
Predicted change in Energy= 2.801952D-07
The optimisation file is linked to here
Vibrational modes
NH4+ is a non-linear molecule with 5 atoms. Using the 3N-6 rule, I expect to see 3(5)-6 = 9 vibrational modes. The frequencies of these 9 vibrational modes are shown below.
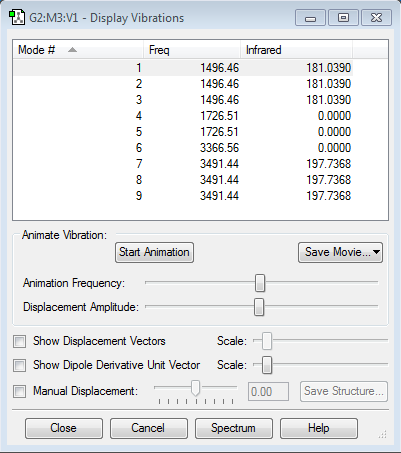
As shown in the table above, some bending and stretching modes have the same frequency, meaning that they have the same energy and are therefore degenerate. Modes 1, 2 and 3 are degenerate, as well as modes 4 and 5. Modes 7, 8 and 9 are also degenerate.
Modes 1, 2, 3, 4 and 5 are "bending" vibrations whereas modes 6, 7 and 8 and 9 are "bond stretch" vibrations. As with ammonia, it can be seen from the table above that "bending" vibrations have much lower frequencies as compared to "bond stretch" vibrations.
Mode 6 is the symmetric stretching of all the N-H bonds. This "bond stretch" vibration does not cause a net change in dipole moment of the tetrahedral ammonium ion, hence it is not detected in IR spectroscopy, resulting in an IR intensity of 0.
However, modes 7, 8 and 9 are assymetric stretches of all the N-H bonds. They cause a net change in dipole moment of the ammonium ion, such that it is larger than the change in dipole moment caused by all the "bending" vibrations. This results in modes 7, 8 and 9 having the greatest IR intensities of 197.7368.
Atomic charges
| Atom | Charge |
|---|---|
| Nitrogen | -0.999 |
| Hydrogen | +0.500 |
Just as in ammonia, there is covalent bonding in the ammonium ion NH4+ between the central nitrogen atom and the hydrogen atoms. As the nitrogen atom is more electronegative than the hydrogen atoms, it pulls the shared electrons in each of the 4 N-H bonds in the ion closer to itself. These N-H bonds are polar covalent bonds. Thus, I expect the nitrogen atom to take on a negative charge and the 4 hydrogen atoms to all take on the same positive charge, which is really the case as shown in the table above. Adding up all the charges of the nitrogen atom and the 4 hydrogen atoms shown above gives 1.001, which shows that the charge of the ammonium ion is indeed +1.
Comparison of atomic charges between NH3 and NH4+
| Atom | Charge in NH3 | Charge in NH4+ |
|---|---|---|
| Nitrogen | -1.125 | -0.999 |
| Hydrogen | +0.375 | +0.500 |
The magnitude of the negative charge on the nitrogen atom is much lower in NH4+ than in NH3. This is because in NH4+, a pair of electrons is donated from the central nitrogen atom to a hydrogen atom to form a dative bond. However, NH3 has a lone pair of electrons on the central nitrogen atom. Thus, there is a higher electron density around the nitrogen atom in NH3 than around the nitrogen atom in NH4+, so the nitrogen atom in NH3 carries a greater negative charge.
However, the hydrogen atoms in NH4+ carry a greater positive charge than the hydrogen atoms in NH3. This is because the electrons in the N-H bonds can be pulled closer to the central nitrogen atom in NH4+ as there is no lone pair-bond pair electronic repulsion in NH4+ when there is lone pair-bond pair electronic repulsion in NH3. Thus, there is a lower electron density around the hydrogen atoms in NH4+ than in NH3, so the hydrogen atoms in NH4+ carry a greater positive charge.
Molecular orbitals
The molecular orbitals of the ammonium ion were visualised using the GaussView software. Images of 5 of these visualised molecular orbitals were captured and saved as files. They are shown below.