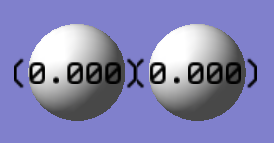Rep:Mod:cp4518
Introduction
Gaussview is a program that was made to draw and analyse key information about molecules and Structures such as General structural information, optimised models of structures, modes of vibrations and charge distribution.
NH3
General Information of optimised NH3
Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -56.55640542 a.u. RMS Gradient Norm 0.00980112 a.u. Imaginary Freq 0 Dipole Moment 1.8466 Debye Point Group C3V
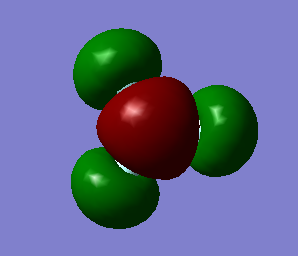
Optimised NH3 molecule |
File link - File:CPeate NH3 optf pop.mol
Optimised bond length of NH3 = 1.03844Å Optimised bond angle of NH3 = 101.721o
Item table
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986272D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Vibrations
| wavenumber cm-1 | symmetry | intensity arbitrary units | image |
|---|---|---|---|
| 1090 | A1 | 145.38 | 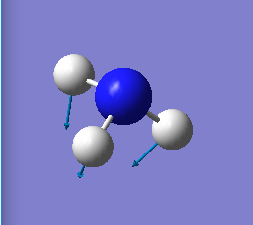
|
| 1694 | E | 13.55 | 
|
| 1694 | E | 13.55 | 
|
| 3461 | A1 | 1.06 | 
|
| 3590 | E | 0.27 | 
|
| 3590 | E | 0.27 | 
|
Questions
In NH3, there are 4 atoms. Therefore, using 3N-6, I expect there to be 6 modes of vibration.
There are two sets of degenerate modes which both contain 2 different vibrations with the same wavenumber, at 1694cm-1 and 3590cm-1. As they both have the same wavenumber they are therefore degenerate and both have the same energy.
NH3 has 3 "bending" vibrations and 3 "bond stretch" vibrations. The "bending" vibrations are at 1090cm-1, 1694cm-1 and again at 1694cm-1. The "bond stretch" vibrations are at 3461cm-1, 3590cm-1 and another at 3590cm-1 (see table of display vibrations for direction of "bending" and "bond stretch" vibrations ).
The vibration at 3461cm-1 is highly symmetrical as all bonds are stretching with same frequency and all have the same displacement from the central nitrogen atom at any given time. The vibration mode at 1090cm-1 is often referred to as the "umbrella" frequency as the 3 H-N bonds bend up and down, mirroring the movement of an umbrella.
For the experimental spectrum of gaseous ammonia you would expect to see 3 bands, one at 1090cm-1, one at 3461cm-1 and another at 3590cm-1. You would not see 6 bands due to degenerate modes of vibration.
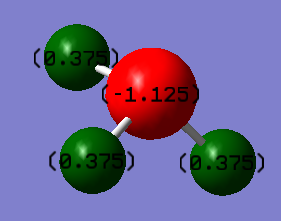
Charge Distribution
Hydrogen:+0.375
Nitrogen:-1.125
The positive charge is on the hydrogen atoms due to its lower electronegativity. Nitrogen has a higher electronegativity and attracts the electron pairs away from hydrogen's, so is therefore negatively charged.
N2
General Information of optimised N2
Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -109.52412868 a.u. RMS Gradient Norm 0.00000365 a.u. Imaginary Freq 0 Dipole Moment 0.0000 Debye Point Group D∞h Bond Length 1.10540 Å
Optimised N2 molecule |
File link - File:CPEATE N2 OPTF POP.mol
Item Table
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000002 0.001800 YES
RMS Displacement 0.000003 0.001200 YES
Predicted change in Energy=-1.248809D-11
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Vibrations
| wavenumber cm-1 | symmetry | intensity arbitrary units | image |
|---|---|---|---|
| 2457 | SGG | 0.00 | 
|
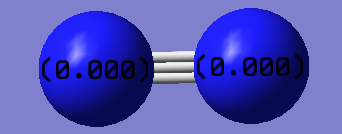
Charge Distribution
As N2 is a homo-nuclear diatomic molecule, both Nitrogen atoms have the same electronegtivity and therefore each atom has a charge distribution of 0.
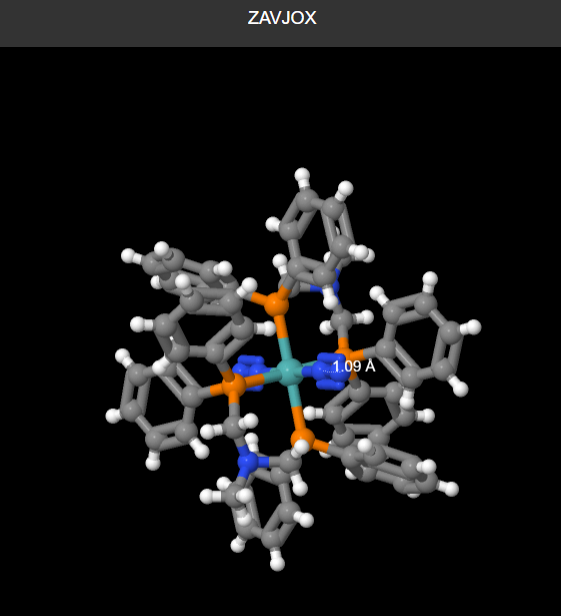
mono-metallic TM complex that coordinates N2
The bond distance that i obtained for N2 was 1.05A while on conquest the bond length was 1.09A. There is a difference in this distance as the computational value depends on the intergration you use. In computational methods the electron density is assumed to be perfectly round where in reality it is slightly different, this difference alters the bond length as the electron repulsion varies slightly from the experimental data.
H2
General Information of optimised H2
Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -1.17853930 a.u. RMS Gradient Norm 0.00012170 a.u. Dipole Moment 0.0000 Debye Point Group D∞H
Optimised N2 molecule |
File link - File:CPEATE H2 OPTF POP.mol
Item Table
Item Value Threshold Converged?
Maximum Force 0.000211 0.000450 YES
RMS Force 0.000211 0.000300 YES
Maximum Displacement 0.000278 0.001800 YES
RMS Displacement 0.000393 0.001200 YES
Predicted change in Energy=-5.852867D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7431 -DE/DX = -0.0002 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Vibrations
| wavenumber cm-1 | symmetry | intensity arbitrary units | image |
|---|---|---|---|
| 4461 | SGG | 0.00 | 
|
Charge Distribution
H2 is a homonuclear diatomic molecule. The charge distribution is 0 as both H atoms have the same electronegativity.
The Haber-Bosch process
energy for the reaction of N2 + 3H2 -> 2NH3
E(NH3)=-56.55640542au
2*E(NH3)=-113.1128108au
E(N2)=-109.52412868au
E(H2)=-1.17853930au
3*E(H2)=-3.5356179au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.05306422au=-139.3kJmol-1
The Haber-Bosch process is exothermic. From my calculated E values, E for NH3 is the most exothermic which results in it being the most stable molecule, more stable than both N2 and H2.
Literature value from experimental data for Haber-Bosch process ΔH(298K)= -45.7kJ/mol
G Austin, Shreve’s Chemical Process Industries, 5th ed., McGraw-Hill International Editions, New York, 1984
NF3
General information of optimised NF3
Calculation Type FREQ Calculation Method RB3LYP Basis Set 6-31G(d,p) Charge 0 Spin Singlet E(RB3LYP) -354.07131066 a.u. RMS Gradient Norm 0.00006772 a.u. Dipole Moment 0.0939 Debye Point Group C3V Bond Length 1.38384 Å Bond Angle 101.864 <sup>o</sup>
Optimised NF3 molecule |
File link - File:Cpeate NF3 optf pop.mol
Item Table
Item Value Threshold Converged?
Maximum Force 0.000082 0.000450 YES
RMS Force 0.000063 0.000300 YES
Maximum Displacement 0.000356 0.001800 YES
RMS Displacement 0.000157 0.001200 YES
Predicted change in Energy=-4.598544D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.3838 -DE/DX = 0.0001 !
! R2 R(1,3) 1.3838 -DE/DX = 0.0001 !
! R3 R(1,4) 1.3838 -DE/DX = 0.0001 !
! A1 A(2,1,3) 101.8637 -DE/DX = 0.0 !
! A2 A(2,1,4) 101.8637 -DE/DX = -0.0001 !
! A3 A(3,1,4) 101.8637 -DE/DX = -0.0001 !
! D1 D(2,1,4,3) 104.9982 -DE/DX = -0.0001 !
--------------------------------------------------------------------------------
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Vibrations
| wavenumber cm-1 | symmetry | intensity arbitrary units | image |
|---|---|---|---|
| 482 | E | 0.52 | 
|
| 482 | E | 0.52 | 
|
| 931 | E | 208.28 | 
|
| 931 | E | 208.28 | 
|
Charge Distribution
Flourine: -0.220 Nitrogen:0.660 Flourine is a more electronegative atom than nitrogen, it pulls the electron pair away from nitrogen and therefore has a negative charge.
computation al power assume radialsphere perfect intergration levevl in computer
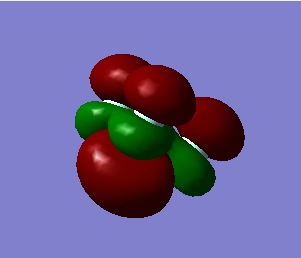
Molecular Orbitals
Marking
Note: All grades and comments are provisional and subjecct to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have recieved your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
NO - you included a .mol file of the optimised structure. As it says in the script we need to have your.log file uploaded. This reduces the achievable mark for this section by 1.
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - You correctly stated that there are two sets of degenerate modes - this explains a spectrum with 4 peaks. However there are only 2 peaks visible as peaks 4, 5 and 6 are of too low an intensity to be visible.
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
NO - you included a .mol file of the optimised structure. As it says in the script we need to have your.log file uploaded. This reduces the achievable mark for this section by 1.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
NO - but you gave the unique identifier.
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 0.5/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
NO - Your final reaction energy is close to the real value of -146.5kJ/mol. This probably stems from incorrect rounding of intermediate results or the use of a wrong conversion factor from Hartree to kJ/mol.
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4.5/5
Have you completed the calculation and included all relevant information?
NO - you included a .mol file of the optimised structure. As it says in the script we need to have your.log file uploaded. This reduces the achievable mark for this section by 1.
Have you added information about MOs and charges on atoms?
You have done a good job of presenting this information, well done! You should have analysed the computed vibration modes as you did for NH3! You correctly stated energies of the MOs. You missed to say for every MO if it is occupied or not. You identified the right AOs contributing to the MOs. However, you missed to include the principle quantum number to make your discussion easier to follow. Except for the last shown MO you correctly identified bonding and anti-bonding characteristics. The last one is noon-bonding as the s-orbitals do not overlap and interact at all!
Independence 0/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far
NO - No independent work has been identified.