Rep:Mod:comp chem lab testpage5
Demo page for the lab
NH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000056 0.000450 YES RMS Force 0.000030 0.000300 YES Maximum Displacement 0.000136 0.001800 YES RMS Displacement 0.000068 0.001200 YES
Frequency file: nh3_frequency.log
Low frequencies --- -29.1845 -27.0206 -27.0196 0.0008 0.0022 0.0076 Low frequencies --- 763.1907 1745.2285 1745.2285
optimised BH3 molecule |
Don't forget that my file is for NH3 and not BH3!
Also note that my lowest frequency is outside of the ±15 cm-1 range!
If this happens to your BH3 molecule, then something is wrong and ask a demonstrator for help!!
In this case, for NH3, the formally zero frequencies are well separated from the lowest energy positive frequency at 1088cm-1 and the large formally zero frequencies are due to the low level of the basis set and relatively relaxed convergence and integration criteria. This can happen for small molecules.
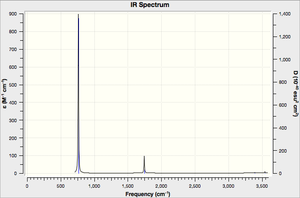
Vibrational spectrum for NH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 763 | 260 | A1 | yes | out-of-plane bend |
| 1745 | 14 | E | very slight | bend |
| 1745 | 14 | E | very slight | bend |
| 3390 | 1 | A1 | no | symmetric stretch |
| 3543 | 1 | E | no | asymmetric stretch |
| 3543 | 1 | E | no | asymmetric stretch |
This is where you add your explanation of why there are fewer vibrational peaks than there are vibrations.(In your wiki explain why are there less than six peaks in the spectrum, when there are obviously six vibrations.)
PP and basis sets
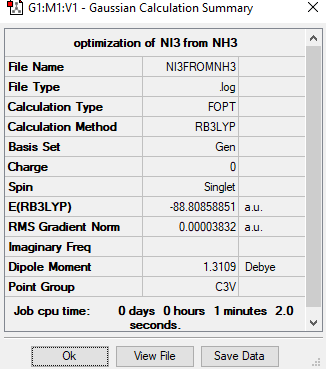
Frequency file: Media:File:6-31G(d,p)LANL2DZ NI3.txt
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000044 0.000300 YES Maximum Displacement 0.000492 0.001800 YES RMS Displacement 0.000333 0.001200 YES
optimised NI3 |
The optimized distance is 2.1836
mini-projects