Rep:Mod:com
reading fc are the best -find 3 additional references
The Hydrogneation of the cyclopentadiene dimer
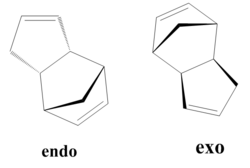
It is possible for two cyclopentadiene dimers to perform a diels alder reaction in order to form one of two isomers, either the endo form or the exo form. The endo and the exo form have different but comparable properties which can be viewed in figure.... If these molecules are hydrogenated they can form either molecule 3 or molecule 4 shown in figure.... It is also possible to form a tetrahydro derivative, however this only occurs after prolonged hydrogenation.
| Energy/kcal mol-1 | endo | exo |
|---|---|---|
| Stretch | 1.25 | 1.29 |
| Bend | 20.85 | 20.59 |
| Stretch-bend | -0.83 | -0.84 |
| Torsion | 9.51 | 7.65 |
| Non-1,4 VDW | -1.52 | -1.42 |
| 1,4 VDW | 4.31 | 4.23 |
| Dipole/Dipole | 0.45 | 0.38 |
| Total Energy | 34.00 | 31.88 |
This table shows that the total energy of the exo form is lower than the total energy of the endo form, however it is known that the endo product is always formed. The difference in total energies can be explained by looking at the breakdown of the individual components. From this it can be seen that the major difference between the exo and endo forms lie in the torsion energy due to the different torsional strain of the rings in each case. In this reaction the kinetic product is formed. This can be explained from the fact that the kinetic product considers the stability of the transition state whilst the thermodynamic product considers the energy of the products.
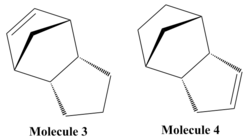
| Energy/kcal mol-1 | Molecule 3 | Molecule 4 |
|---|---|---|
| Stretch | 1.30 | 1.09 |
| Bend | 19.81 | 14.52 |
| Stretch-bend | -0.84 | -0.55 |
| Torsion | 10.82 | 12.51 |
| Non-1,4 VDW | -1.20 | -1.07 |
| 1,4 VDW | 5.63 | 4.50 |
| Dipole/Dipole | 0.16 | 0.14 |
| Total Energy | 35.64 | 31.16 |
XML error: Mismatched tag at line 1
