Rep:Mod:coke1
Organic Computer Lab
All energies are quoted to 2 d.p and all calculations are carried out using Allinger MM2 molecular mechanic models (1) through ChemBio 3D unless otherwise stated
The Hydrogenation of Cyclopentadiene Dimer
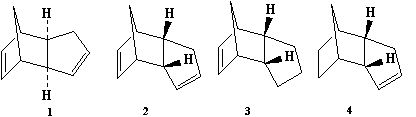
When cyclopentiene dimerises through a [4+2] cycloaddition there are two possible products, 1 and 2. It is known that that 2 is the overall product with the hydrogenation of the subsequent cyclopentadiene gives the isomers 3 or 4.
When looking at the isomers 1,2,3 and 4 the following results are seen when minimising the energies when using MM2 on chembio 3D
| Energy kcal/mol | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Total energy | 31.88 | 34.01 | 34.96 | 29.25 |
| Stretch | 1.29 | 1.24 | 1.27 | 1.12 |
| Bend | 20.58 | 20.86 | 19.09 | 13.02 |
| Torsion | 7.67 | 9.50 | 11.14 | 12.42 |
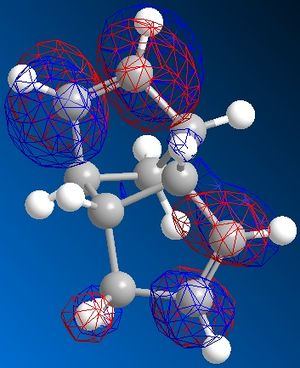

It is clear to see that 1 and 4 are the most thermodynamically stable of the 1/2 and 3/4 pairings. This would suggest that when cyclopentiene dimerises it does not go through the thermodynamically stable route but instead is kinetically controlled. It can be easily seen why 1 is thermodynamically more stable than 2 through looking at the dihedral angle between the bridging over the 6-membered ring and the 5-membered ring. In 1 this angle is 148 o but with 2 it is 75 o With the molecule wanting to reduce the steric interactions between the large groups this leads to the groups wanting to be further away from each other which is obtained from orientation 1. This repulsion between groups can be seen quantativly from the torsion energy difference between the two isomers.
The stability of 2 and thus why the reaction proceeds through this route can be explained through the exploration of the electronic structure of the cyclopentaidne with the endo having a more stable transition state when looking at orbital overlap. It can be considered that to produce 1 the orbitals are "in-line", this creates a situation where all the other orbitals are also in the same plane and able to contribute to non-bonding thus making it a less stable transition state than 2 where all the orbitals are not so in line and all the orbitals are in the correct phase to create a bond.
When looking at 3 and 4 there are some aspects of their energy distribution that are quite similar such as their stretching, torsion and Van Der Waals energies. The main differences are in the bend energies with 4 being 5kcal/mol less then 3 thus in a thermodynamic environment 4 would be the preferred product. When looking at the angles involved in the bonding it can be seen that there is more bending strain in 3. A focus on the sp 2 carbon in the double bonds involved it is seen that the double bond in the 6-membered ring has an angle of 107 o where as the 5 membered ring has an angle of 124 o, with the angle of an optimum sp 2 carbon being 120 o, by hydrogenating the 6 membered ring much more strain is relieved and thus is more thermodynamically favorable.
This can also be looked at from a molecular orbital approach looking at the HOMO and LUMO of the molecule before the hydrogenation. When looking at the HOMO the electron density over both bonds is of a similar size but looking the LUMO it can be seen that there is much larger empty orbitals around the double bond in the bridged 6-membered ring which could potentially be used when complexing with the metal complex during hydrogenation. This makes this bond more susceptible to hydrogenation which is what is seen. (calculations performed on Mopac interface)
When looking at the actual result from the hydrogenation of dicyclopenadiene (using H2 and a Ni raney catalyst) the product is predominately 3 showing that the this reaction is under kinetic control (this was obtained taking the product as the intermediate from the reaction of dicyclopentadiene to the fully hydrogenated form). (2) But it should be noted that by changing the reaction conditions is is easy to change this to thermodynamic control. (3)
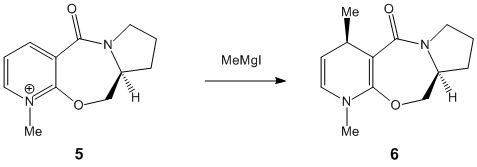
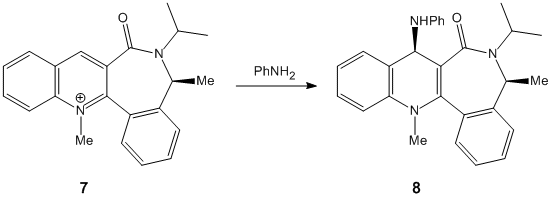
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue).
For these reactions only the carbonyl compound was investigated with Chembio 3D as the computer program was unable to program through heavier atoms in the MeMgI and therefore no results were able to be obtained. The following two reaction schemes were investigated;

The first point of the investigation was to locate the geometry of the carbonyl on the reactants and therefore see what effect this had on the attack on the 1,3-position on the aromatic compound. The geometry was tested with different starting locations of the substituent groups- the ones quoted below are the optimized calculations. The total energies were as follows;
5- 44.3 kcal/mol
7- 62.7 kcal/mol
The aromatic ring was set with their x coordinates being 0 which gave the coordinates of the numbered atoms below (1 being the carbonyl oxygen, 2 being the carbonyl carbon, 3 being the aromatic carbon alpha to the carbonyl, 4 being the beta carbon in the aromatic ring) and dihedral angle of the carbonyl oxygen to the aromatic ring being;
5- dihedral angle -12 o
7- dihedral angle 8 o
| atom for reactant 5 | x | y | z | atom for reactant 7 | x | y | z | |
|---|---|---|---|---|---|---|---|---|
| 1 | -0.43 | 3.37 | 0.59 | 1 | -0.52 | -2.95 | -1.58 | |
| 2 | -0.014 | 2.68 | -0.32 | 2 | -0.08 | -2.69 | -0.45 | |
| 3 | -0.00 | 1.33 | -0.16 | 3 | 0.00 | -1.35 | -0.13 | |
| 4 | -0.00 | 0.49 | -1.23 | 4 | -0.01 | -0.78 | 1.09 |
As can be seen above in the molecular diagrams, 5 has the carbonyl above the aromatic ring i.e. the same side as the Me attack and in 7 the carbonyl oxygen is below the aromatic ring i.e. on the opposite side to the face of attack. In both molecules the R group (R= Me 6 R= NHPh 8) is added to the "top" face of the molecule. This would suggest that there are two different mechanisms involved in these attacks of the aromatic ring next to the carbonyl carbon. When firstly looking at the addition of MeMgI to reactant 5 it could be proposed that there is an interaction between the Mg atom within the MeMgI and the oxygens lone pair. This would be due to the electropositive nature of the metal centre. This interaction weakens the Me-MgI bond and makes the Me within the molecule more electronegative. Due to the Mg interacting with the oxygens lone pair the Me is forced to interact on the same face due to the bond length providing too much strain to attack from the opposing face. This is why the Me group attacks the same face as the oxygen even though this provides the molecule with more steric interaction.(3)
In reactant 7 there is a much larger dihedral angle leading to much more sterics involved on the face that it occupies. Within this reaction scheme there is no electropositive site to be stablised by the oxygens lone pair. The lone pair in the oxygen instead adds to the steric bulk of the oxygen making it larger and creating more steric hindrence. Instead the attacking group- being large in size- is repelled from the face which the carbonyl oxygen is on and attacks from the reverse face where these steric factors are reduced.
This simple model could be improved by including the metal, Mg, complexing with the oxygen as this program is not capable of computing Mg as an atomic centre when looking at molecular mechanics. So by editing the program to include this interaction a better representation of the molecule could be gained and this would provide a better idea of the strains and energy of the molecule. There is also the fact that we are only computing it to MM2 so by increasing the complexity of the molecular mechanics that we are using a better representation would be gained.
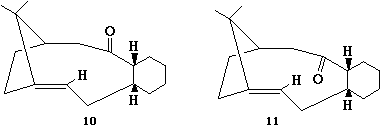
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol.
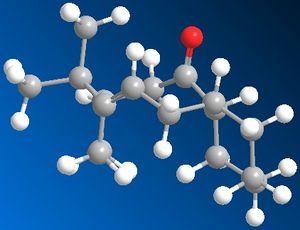
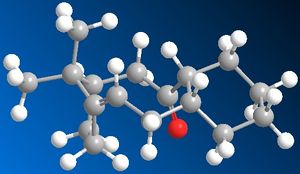
10 and 11 are intermediates in the reaction scheme for Taxol. Their stability was calculated using MM2 on chembio 3D giving the following results after moving the molecule around for several attempts to find the optimum structure.
10 -44.30 kcal/mol
11 - 48.90 kcal/mol
This indicates that 10 is more stable with a lower energy overall. It should also be noted that this alkene reacts comparatively slowly with further functionalisation. These two observations can be explained through firstly looking at the orientation of the alkene hydrogen and the carbonyl oxygen, in 10 it is seen that they are in the right orientation to undergo a weak hydrogen bonding interaction creating more stability for the molecule which would be lost if the alkene were to undergo further functionalisation. In 11 this orientation isn't as strong as the atoms are further away from each other, so it does not gain stability this way. It should also be noted that both 10 and 11 show unusually slow reaction to further functionalisation, this is due them being hyperstable alkenes due to the position of the olefin in relation to the bridging. To show this we look at the "olefin strain" energy which is defined as the strain energy between the parent hydrocarbon and the olefin in question. With a hyperstable alkene the position of the alkene with relation to the bridging means that the strain of the parent molecule is higher then the olefin thus reducing the reactivity of the olefin. It can be seen that this is the most thermodynamically stable position of the olefin within the molecule. (5)
Regioselective Addition of Dichlorocarbene
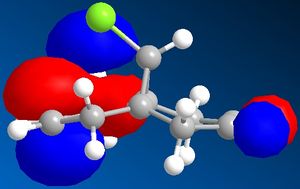

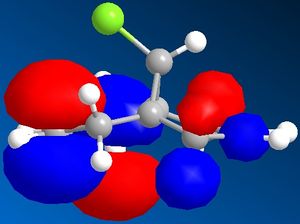
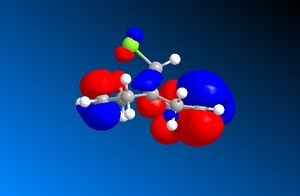
When looking at dichlorocarbene, the molecules energy was first minimised by MM2 calculations but then further minimized using MOPAC/PM6 methods which produced the orbital approximations seen. Here are the projections of the LUMO and HOMO, the numbering indicated how close they are to the HOMO/LUMO boundary- 1 being the highest filled/ lowest unfilled orbital. It is clear to see that the program has taken into account the rigidity of the molecule and is therefore treating the two double bonds as two separate environments by the orbitals of the double bonds being of different shape and size.
When looking at which bond is more nucleophilic (most susceptible to electrophilic attack)the focus was on the HOMO 1. There is a larger HOMO 1 orbital over the bond syn to the C-Cl bond ("under" the C-Cl bond), this incresed electron density over the double bond makes it more nucleophilic and therefore more reactive.
When focusing on the vibrational aspect of the molecule and the effect that the Cl-C bond has on the molecule the following vibrations were seen

| Assignment | Diene Frequency/ cm -1 | intensity | monoene Frequency/ cm -1 | intensity |
|---|---|---|---|---|
| =C-H bending | 689 | 53 | 695 | 34 |
| C-Cl | 770.8 | 25 | 764 | 18 |
| C-H alkane stretch | 3000-3033 | 10-80 | 3006-3097 | 13-81 |
| =C-H stretch (1) | 3153 | 7 | 3158 | 9 |
| =C-H stretch (2) | 3156 | 6 | - | - |
| =C-H stretch (1) | 3176 | 54 | 3185 | 56 |
| =C-H stretch (2) | 3178 | 35 | - | - |
The monoene observed had the double bond anti to the C-Cl bond hydrogenated. Both molecules have C1 symmetry but have different dipole moments with the monoene having the larger dipole moment of 2.84 debyes and the diene having a dipole moment of only 2.21 debyes.
It can be seen that through these values there are 2 distinct alkene peaks for the dimer whereas there is only 1 for the monoene. There is also a small change in the wave number for these =C-H stretch from the monoene and the diene, with an increase of over 4 cm -1from diene to the monoene. This increase in wave number signifies a stronger bond which is seen in the monoene. This is what is expected as the hydrogenation of the double bond would release some of the strain on the ring that the double bond incurs. The C-Cl bond in both molecules are similar energy but the diene is slightly stronger. This would be due to the increased in strength in the double bond in the monoene. With this increase in orbital size in the double bond "under" the Cl, there would hold more electron density closer to the Cl. This would destablise the bond through sterics and electron donation from this pi orbital into the sigma * of the C-Cl bond.
Mini project using DFT-based Molecular orbital methods
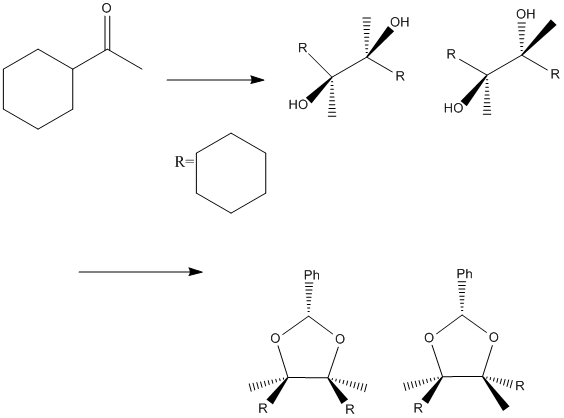
For the mini project I have chosen the molecule (4RS,5RS)- and (2R,4RS,5SR)-4,5-Dicyclohexyl-4,5-dimethy-2-phenyl-1,3-dioxolane. This molecule was obtained from the meso- and dl-2,3-dicyclohexyl-2,3-butanediol which in itself was obtained from reaction 2 equivalents of actyl cyclohexane in the presence of a low valence metal.(6) The reaction scheme below is not stoichiometric but indicates where the differnece in geometry originates from. For full synthesis see the literature reference above. In the diagram below the (2R, 4RS, 5SR) is on the left and the (4RS, 5RS) is on the right. For the first part of the reaction there is a preference for the dl- product (left intermediate) to the meso- product (right intermediate) when using a low valent metal to catalyse the reaction. This would be due to the metal centre complexing with both oxygen’s forcing them to be on the same face as the reaction proceeds. The reaction still does not proceed to just that one isomer though, as the reaction is a radical reaction so both are mechanistically plausible and occur.
| Peak from literature/ppm | Peak from Computation/ppm | Assignment | Difference/ppm |
|---|---|---|---|
| 14.7 | 17.3 | 9 | 2.6 |
| 17.6 | 20.5 | 8 | 2.9 |
| 26.6 | 27.5 | 14 | 0.9 |
| 26.7 | 27.8 | 19 | 1.1 |
| 26.9 | 28.0 | 18 | 1.1 |
| 27.1 | 28.1 | 20 | 1.0 |
| 28.6 | 28.3 | 13 | 0.3 |
| 28.8 | 28.7 | 38 | 0.1 |
| 29.9 | 29.0 | 15 | 0.9 |
| 30.3 | 30.3 | 17 | 0 |
| - | 31.5 | 33 | - |
| - | 32.6 | 12 | - |
| 42.9 | 42.9 | 10 | 0 |
| 44.0 | 44.5 | 7 | 0.5 |
| 87.9 | 92.0 | 3 | 4.1 |
| 88.0 | 92.3 | 2 | 4.3 |
| 98.7 | 103.1 | 5 | 4.4 |
| 126.6 | 122.2 | 25 | 4.4 |
| - | 122.8 | 21 | - |
| 128.1 | 124.3 | 23 | 3.8 |
| 128.6 | 124.6 | 22 | 4 |
| - | 124.8 | 24 | - |
| 139.8 | 140.3 | 6 | 0.5 |
| Peak from literature/ppm | Peak from Computation/ppm | Assignment | Difference/ppm |
|---|---|---|---|
| 16.1 | 18.8 | 8 | 2.7 |
| - | 18.8 | 9 | - |
| 25.9 | 27.7 | 20 | 1.8 |
| 26.0 | 28.0 | 19 | 2.0 |
| 26.1 | 28.0 | 14 | 1.9 |
| 26.4 | 28.1 | 18 | 1.7 |
| 26.5 | 28.3 | 13 | 1.8 |
| 26.6 | 28.4 | 15 | 1.8 |
| 28.3 | 29.6 | 33 | 1.3 |
| 28.7 | 30.3 | 17 | 1.6 |
| 29.9 | 30.8 | 38 | 0.9 |
| 31.5 | 31.3 | 12 | 0.2 |
| 49.1 | 42.1 | 10 | 7.0 |
| 51.8 | 42.6 | 7 | 9.2 |
| 87.4 | 91.8 | 2 | 4.4 |
| - | 92.1 | 3 | - |
| 99.0 | 101.1 | 5 | 2.1 |
| 126.3 | 121.9 | 25 | 4.4 |
| - | 122.9 | 21 | - |
| 128.0 | 124.2 | 23 | 3.8 |
| 128.3 | 124.7 | 22 | 3.6 |
| - | 124.8 | 24 | - |
| 139.8 | 140.0 | 6 | 0.2 |
( C13NMR not stated in usual format for ease of comparison and dual assignment)
The C13 NMR is with reference to CDCl3
The root mean square error was 1.94 ppm for the (4RS,5RS)isomer and 2.75ppm for the (2R,4RS,5SR)isomer.It can be seen that there is good agreement with the literature value and the computational values with most only being out by 1-2 ppm- almost all are within the 5ppm cut off point for obtaining the correct geometry.The only 2 carbon atoms which are outside the 5ppm range are C7 and C10 (the carbons connecting the cyclohexane groups the dioxolane) in the (2R,4RS,5SR)isomer. Reverting back to the first steps, the carbon atoms in question were manually moved within the following 2 constraints;
The carbons were kept tetrahedral
The overall geometry was kept the same and as specified in the diagram in the start
After the manual movement of these carbon atoms the MM2 geometry optimisation would only give the geometry of the initial molecule (the set of coordinates for the molecule was the same as the ones already computed) and therefore subsequent optimisation would be to the same minima energy. Therefore it can be seen that there may be a slight variation in the geometry of these two carbons from the literature geometry although all the rest of the carbons are in agreement.
Some of the peaks seen in the computational analysis are not seen in the literature values stated in the report- this would be due to the computational program not taking into consideration the thermal contributions of the molecule in its geometry. This would mean that in the "real" NMR these extra peaks would not be seen as individual peaks but more likely a broadened peak centered around their average. The other reason there may be more peaks in the computational NMR spectra would be down to symmetry. In the computer program it does not account for carbon atoms being in the same enviroment. This means that in the aromatic ring where there is a defined line of symmetry it has slightly separated the peaks for atoms which, in a real life NMR, would be only 1 peak.i.e peak for carbon 8 and 9 in (2R,4RS,5SR)-4,5-Dicyclohexyl-4,5-dimethy-2-phenyl-1,3-dioxolane where the separation in the peaks is less then 0.05 but is still stated as 2 peaks on the computation output.
Experimentally this reaction was also analysed at the step before the two molecules that I am analysing where sythesised. This analysis means they are able to purify a step eariler to prevent there being a mixture for the end product- however doing the reaction scheme all in one vessel a mixture of the products would be obtained. Assignments were made by comparing the relative heights of methyl signals at d 1.14 and d 1.15, respectively on the H- NMR spectra. These peaks were used as it is the methyl that would show the largest difference between the 2 isomers. By this method they were able to verify that they had obtained the correct products at each stage. The reaction mixture was filtered and evaporated. Thin-layer chromatography was then used to separate the products and produce the 2 isomers in pure separate solutions.
| Assignment | literature/ cm -1 | 4RS,5RS Frequency/ cm -1 | intensity | error/ % | literature cm -1 | 2R,4RS,5SR Frequency/ cm -1 | intensity | error/ % |
|---|---|---|---|---|---|---|---|---|
| C-H | 3062 | 3076 | 108 | 0.46 | 3008 | 3076 | 110 | 2.26 |
| ALPHA H TO BOTH CARBONYLS | 2954 | 2964 | 76 | 0.34 | 2924 | 3017 | 24 | 3.18 |
| =C-H | 2854 | - | - | - | 2854 | 2961 | 63 | 3.74 |
| C=C aromatic | 1689 | 1537 | 6 | 8.99 | 1682 | - | - | - |
| C=C aromatic | - | - | - | - | 1612 | - | - | - |
| C-H | 1450 | 1416 | 14 | 2.34 | 1450 | 1428 | 21 | 1.51 |
| ALPHA H TO BOTH CARBONYLS | 1381 | 1394 | 89 | 0.94 | 1373 | 1397 | 108 | 1.74 |
| C-H | 1296 | 1239 | 5 | 4.39 | 1242 | 1238 | 59 | 0.32 |
| C-C | 1103 | 1110 | 110 | 0.63 | 1141 | 1120 | 190 | 1.84 |
| C-C | 1072 | 1095 | 10 | 2.14 | 1095 | 1106 | 56 | 1.00 |
| C=C AROMATIC | 1026 | 1055 | 20 | 2.82 | 1025 | 1032 | 41 | 0.68 |
| C-H | 926 | 950 | 14 | 2.59 | 926 | 951 | 13 | 2.69 |
| C-H ALKANE | 887 | 901 | 6 | 1.57 | 849 | 887 | 9 | 4.47 |
| -C-O AND C-H | 764 | 777 | 18 | 1.70 | 756 | 734 | 37 | 2.9 |
| -C-O AND C-H AROMATIC | 710 | 732 | 34 | 3.09 | - | - | - | - |
The error is the percentage error for each of the values given.
The IR shows slightly less agreement then the NMR but the overall form of the IR is very similar and shows that the strength of the bonding is similar to the literature values. All of the values are below 10% which gives a good overall agreement. There has to be consideration for the fact that the computer programme runs the IR analysis in gas phase where as literature values are not stated as gas phase. This agreement supports the NMR assignment. It can be seen that these two spectrum do support the structural assignment in the literature. Overall the sum of the free energies was seen to be
(4RS, 5RS)= -1046.94 Hartrees
(2R,4RS,5SR)= - 1046.95 Hartrees
This shows there is little difference (6.3 kcal/mol) in the overall energies of the two isomers even though they have high rigidity and the isomers are not interconvertable. The optical rotation and CD (Circular Dichroism) spectrum were not quoted in the literature so have not been calculated here.
References
(1)E. Bright Wilson, Jr. and Bryce L. Crawford, Jr. J. Chem. Phys. 6, 223 (1938); doi:10.1063/1.1750232
(2) Ji-Jun Zoua, Xiangwen Zhanga, Jing Konga and Li Wang, Fuel, Vol 87, 17-18, Dec 2008,pp 3655-3659 doi:10.1016/j.fuel.2008.07.006
(3) D. Skala and J. Hanika, petroleum and coal, vol 45, 3-4,pp 105- 108
(4) Handbook of grignard Reagents, Gary S. Silverman, Philip E. Rakita, Marcel Dekker, New York, 1996 pp 188-189
(5) Wilhelm F. Maier, Paul Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103 (8), pp 1891–1900 DOI: 10.1021/ja00398a003
(6) Akifumi Kagayama, Koji Igarashi, and Teruaki Mukaiyama Can. J. Chem. 78(6): pp 657–665 (2000) doi:10.1139/cjc-78-6-657
Data
NMR 4RS 5RS
http://hdl.handle.net/10042/to-2532
NMR 2R 4RS 5SR