Rep:Mod:coffee table
Optimisation of BH3 via Gaussian
B3LYP/6-31G level
Result summary table

Convergance
Item Value Threshold Converged? Maximum Force 0.000046 0.000450 YES RMS Force 0.000023 0.000300 YES Maximum Displacement 0.000182 0.001800 YES RMS Displacement 0.000091 0.001200 YES
Frequency analysis
Log file
Low frequencies --- -0.4072 -0.1962 -0.0054 25.2514 27.2430 27.2460 Low frequencies --- 1163.1897 1213.3128 1213.3155
Although the low frequencies is out of the range of 12 ± 15 cm-1, the calculation has converged, the geometry and the total energy is correct and I have discussed with the demonstrator who confirmed that it is not outstandingly concerning.
Frequency table
| Mode number | frequency (cm-1) | Intensity | Symmetry | type |
|---|---|---|---|---|
| 1 | 1163 | Strong | A1 | Bend |
| 2 | 1213 | weak | E | Scissoring |
| 3 | 1213 | weak | E | Rocking |
| 4 | 2582 | not observed | A1 | Symmetric stretch |
| 5 | 2715 | strong | E | Asymmetric stretch |
| 6 | 2715 | strong | E | Asymmetric stretch |
Predicted IR spectrum
Smf115 (talk) 17:06, 28 May 2018 (BST)Correct bond assignment for the vibrational modes. However, the symmetries are incorrect suggesting and no explanation is given for why only three peaks are visible in the spectrum.
3D model
Optimised BH3 |
Molecular Orbitals

The diagram above demonstrates that LCAOs using MO theory predicts the nodes between the orbital lobes and the energy levels accurately though does not predict how diffused the orbitals are and the actual extent of how much the AOs overlap each other. Qualitative LCAOs are therefore useful to predict the energy level and the location of the nodes but not the actual shape of the orbitals itself and how diffused they are.
NH3 analysis
B3LYP/6-31G level
Result summary table

Convergance
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000039 0.001800 YES RMS Displacement 0.000013 0.001200 YES
Frequency analysis
Log file
Low frequencies --- -8.5646 -8.5588 -0.0047 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Vibrational modes and intensities

3D model
Optimised NH3 |
NH3BH3
B3LYP/6-31G level
Result summary table

Convergance
Item Value Threshold Converged? Maximum Force 0.000117 0.000450 YES RMS Force 0.000063 0.000300 YES Maximum Displacement 0.000839 0.001800 YES RMS Displacement 0.000362 0.001200 YES
Frequency analysis
Log file
File:Coffeetable nh3bh3 freq.log
Low frequencies --- 0.0004 0.0006 0.0013 18.5187 24.9168 40.9948 Low frequencies --- 266.3754 632.3711 639.9574
Vibrational modes and intensities

3D model
Optimised NH3BH3 |
B-N Bond strength in NH3BH3
Energy Calculation
To get the association energy of BH3 and NH3, the total energy of the caclulations are substituted into this equation:
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
The calculated total energies are:
BH3= -26.61532 a.u.
NH3= -56.55776 a.u.
NH3BH3= -83.22469 a.u.
Substituting into the equation:
ΔE=-83.22469-[-56.55776-26.61532]= -0.05161 a.u. ≈ -136 kj/mol
The association energy of NH3BH3 is therefore very weak. On average, the C-C bond energy is -347 kj/mol which is a lot higher than that of NH3BH3. To put in perspective, compare with a very weak bond such as F-F which has the association energy of -154 kj/mol[2], the association energy is still higher in NH3BH3 which shows how weak it is.
Smf115 (talk) 17:05, 28 May 2018 (BST)Correct calculation and accuracy of the reported energies. Good comparison to evaluate the strength of the bond with referenced literature values.
Psuedo-potential optimisation for BBR3 via B3LYP/6-31G(d,p)LANL2DZ
Result summary table

Convergance
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000018 0.001200 YES
Frequency analysis
Log file
File:Coffeetable bbr3 freq.log
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Vibrational modes and intensities

3D model
Optimised BBr3 |
Dspace Link/unique identfier
Day 2: Analysis of aromaticity
Benzene
Result summary table
Convergance
Item Value Threshold Converged? Maximum Force 0.000198 0.000450 YES RMS Force 0.000087 0.000300 YES Maximum Displacement 0.000757 0.001800 YES RMS Displacement 0.000321 0.001200 YES
Frequency analysis
Log file
File:Coffeetable benzene freq.log
Low frequencies --- -2.1456 -2.1456 -0.0089 -0.0045 -0.0043 10.4835 Low frequencies --- 413.9768 413.9768 621.1390
Vibrational modes and intensities

3D model
Optimised benzene |
Borazine
Result summary table
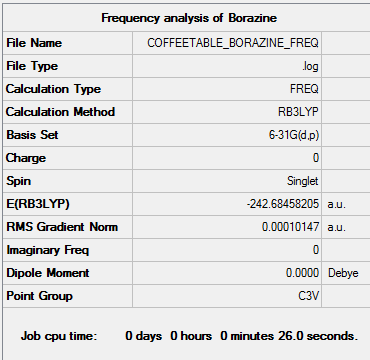
Convergance
Item Value Threshold Converged? Maximum Force 0.000313 0.000450 YES RMS Force 0.000101 0.000300 YES Maximum Displacement 0.000511 0.001800 YES RMS Displacement 0.000151 0.001200 YES
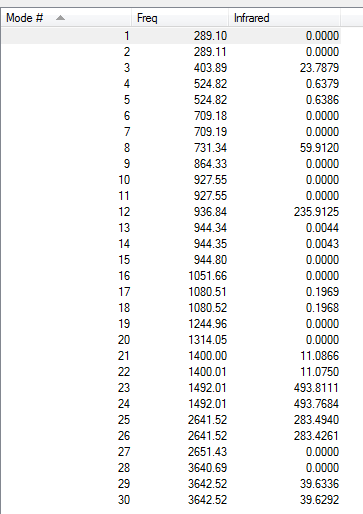
Frequency analysis
Log file
File:Coffeetable borazine freq.log
Low frequencies --- -12.2781 -12.1074 -8.6374 -0.0099 -0.0086 0.0754 Low frequencies --- 289.0994 289.1102 403.8930
Vibrational modes and intensities
3D model
Optimised borazine |
Comparison between benzene and borazine
Charge distribution
The charge distribution analysis was performed on both the compounds with these settings:
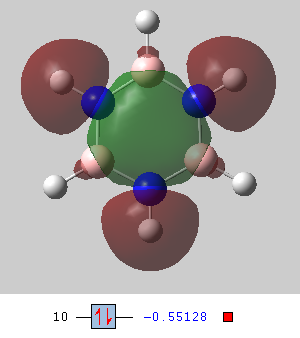
Molecular orbitals
Smf115 (talk) 15:06, 1 June 2018 (BST)Nice selection of comparable MOs and good comparison with clear identification of the character for each MO. To improve, when discussing the symmetry of the orbitals consider including details such as the point group of the orbital or the relevant symmetry elements.
The concept of aromaticity
Aromatic compounds are especially stable compounds when compared to their related structures and was first observed in benzene. The concept of aromaticity has evolved throughout the years since Kekule's definition on benzene, deducing that any compound related to benzene is aromatic. Another common definition of aromatic compounds are cylic compounds that have delocalised pi electrons, planar and follow Hückel's Rule of 4n+2 electrons. The current definition of aromaticity defines that for any compound to be aromatic, it must follow these criteria:
1) They must be more stable than the acyclic conjugated analogue compound or the saturated cyclic analogue compound. Therefore even if the compound has overlapping pz AOs in a linear conjugated system, it isn't necessarily an aromatic compound. Similarly, some cyclic compounds that have overlapping pz AOs are not stablised, even destablised by the system (anti-aromatic) thus these compounds are not aromatic; overlapping pz AOs is therefore not a good description for aromaticity.
2) They have bond lengths between the typical bond length values of single and double bonds; this is because the bonds in aromatic/resonance stablised compounds have the bonding molecular orbitals somewhat in between single and double bonds.
3) When subjected to a magnetic field, a pi-electron ring current is induced which in turn induces it's own magnetic field that counters the external magnetic field leading to the higher diamagnetic susceptibility values in 1H NMR spectroscopy for hydrogens that are outside the ring. Linear conjugated compounds do not exhibit this effect.
4) Under go chemical reactions where the pi-electron conjugated structure is conserved (aromatic substitution reactions).
Since aromaticity was first observed with benzene and other planar molecules like naphthalene, classically all aromatic compounds must be planar but following the modern definition, some non-planar compounds such as cyclophanes exhibit aromatic properties. Much of the aromaticity of a compound can be accounted to the cyclic pz overlapping AOs [3].
Although much of the effects can be accounted to the delocalised pi system, it is debated that other p and s-electron orbitals may play a role in the stability of aromatic compounds. Some compounds exhibit aromatic properties despite the lack of pi-electron structures and the aromaticity is purely due to sigma-electron structures. Examples of these compounds are trihydrogen cations ([H3]+) and PtZnH5- anion that, dubbed to be σ-aromatic [4]. The concept of aromaticity thus is still debatable and will likely change to account more observations and phenomena.
Smf115 (talk) 15:09, 1 June 2018 (BST)A clear discussion of the main concepts of aromaticity and reference made to the MOs just viewed. However, the ideas raised to illustrate why overlapping pZ AOs are a bad descriptor of aromaticity could be developed further and other MOs could be used to illustrate the points made, such as sigma-aromaticity.
Smf115 (talk) 15:09, 1 June 2018 (BST)Overall, a good report with clear use of terminology when evaluating the MOs.
Reference
- ↑ http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf
- ↑ http://butane.chem.uiuc.edu/cyerkes/Chem104ACSpring2009/Genchemref/bondenergies.html
- ↑ DOI: 10.1002/chem.200700250
- ↑ DOI: 10.1021/jz500322n