Rep:Mod:cm3414
Transition States Computational Lab
Introduction: Transition States
A transition state is an instantaneous state that molecules go through during a reaction between products and reactants; it cannot be detected by spectroscopic methods. In the context of a potential energy surface, it is a saddle point, the highest energy point (maximum) on the surface, with a gradient of zero (i.e. dy/dx = 0) and a negative curvature (i.e. d2y/dx2 =-ve)[1]. A minimum is the lowest energy point on a potential energy surface and corresponds to physically stable chemical species, with a gradient of zero (i.e. dy/dx = 0) and a positive curvature (i.e. d2y/dx2 =+ve).
In this computational lab, the programme GaussView was used to create, optimise, and carry out transition state, IRC and frequency calculations on reactions to follow the progress of reactions, determine transition state energies and deduce the most favourable outcomes of reactions.
Nf710 (talk) 10:23, 30 January 2017 (UTC) Your definition of a TS is incorrect. A TS have positive curvature in every co ordinate apart from the reaction coood which is negative.
Exercise 1: Reaction of Butadiene with Ethylene
The reaction between Butadiene and Ethylene is a Diels-Alder reaction which proceeds via a cyclic transition state, as shown below in Scheme 1:

Molecular Orbitals
The MO diagram for this reaction, including basic symmetry labels, is shown below in Fig. 1. In this reaction the diene (butadiene) LUMO and dieneophile (ethene) HOMO are close in energy, which results in a bonding interaction between the two and forms the HOMO and LUMO of the TS:

That these orbitals interact with one another in this way is confirmed by visualisation of the MOs in Gaussview. The HOMOs and LUMOs of ethene and butadiene are shown below, which correlate to those shown on the MO diagram above:

Visualisation of the TS MOs allows them to be correlated to those shown in the MO diagram. Below the TS HOMO and LUMO are shown, which, as illustrated, clearly correlate to the in-phase and out-of-phase interaction of the diene LUMO and dieneophile HOMO:


This confirms that it is indeed the dieneophile HOMO and diene LUMO which are closest in energy and interact to from the TS HOMO and LUMO.
From the MO diagram shown above in Fig. 1, conclusions can be made about the symmetry requirements of a reaction. Only those orbitals which are of the same symmetry, i.e. both symmetric or both asymmetric, can give an allowed reaction. An interaction between a S and AS orbital is forbidden. This is because the overlap integral for an AS-AS or S-S interaction is non-zero, whereas that for an AS-S interaction is zero.
Bond Lengths
The progression of the reaction can be viewed in terms of the changing bond lengths. Typical C-C bond lengths are shown in Table 1[2].
| sp3-sp3 | sp3-sp2 | sp2-sp2 |
|---|---|---|
| 1.45 | 1.50 | 1.33 |
The changes in bond lengths as the Diels-Alder reaction between ethene and butadiene are shown below in Fig. 5 from the reactants, through the transition state, and to the products.
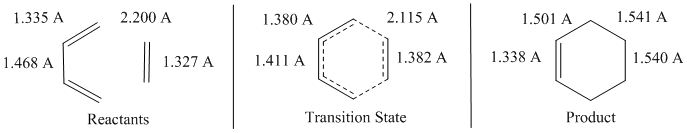
It can be seen that as the reaction progresses, the double bonds in butadiene change to single bonds and lengthen from 1.335-1.501 A. The single bond in butadiene transforms to a double bond and shortens from 1.468-1.338 Å. The bond length of ethene also increases, from 1.327-1.540 Å. The reactants were initially separated by 2.200 Å where new C-C bonds would form, and this shortens to 1.541 Å as the bonds form. These bond lengths roughly match those expected both in the products and reactants, with the bond lengths in the transition state all being intermediate between the expected lengths in the reactant and product. The Van der Waals radius of a C atom is 1.7 Å[3], which means that a bonding interaction would occur between C atoms which are less than 2.4 Å apart, indicating that in the TS the newly forming C-C bonds are definitely experiencing a bonding interaction.
Transition State Vibrations
The vibration shown below in Fig. 6 is that which corresponds to the reaction path at the transition state. In Gaussview it appears as an imaginary vibration at -948.48. It shows that the formation of the two bonds is synchronous.

Note: for an unknown reason wiki would not upload the animated vibration gifs, however the file can be viewed here: https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:TS_Imaginary_Vibration_cm3414.gif
Conversely, the lowest positive (i.e. real, not imaginary) frequency is 145.12 and is asynchronous, with the molecule of ethene twisting towards one end of the butadiene molecule:

Note: for an unknown reason wiki would not upload the animated vibration gifs, however the file can be viewed here: https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:TS_lowest_positive_vibration_cm3414.gif
Nf710 (talk) 10:33, 30 January 2017 (UTC) This was a good sections, you could have shown more theoretical understanding such as weather it is a normal or inverse electron demand reaction.
Exercise 2: Reaction of 1,3-Diaxole with Cyclohexadiene

(In this image, endo and exo are the wrong way around Tam10 (talk) 17:40, 27 January 2017 (UTC))
MO Diagram
The HOMOs and LUMOs of 1,3-dioxole and cyclohexadiene are shown below, and beneath that are the HOMOs and LUMOs of the transition states in both the endo and exo products. The MO diagram of this reaction is similar to that shown in Figure 2, with 1,3-dioxole acting as the diene and cyclohexadiene the dieneophile.


(Double check your wiki, the same image is used twice. The correct image is here Tam10 (talk) 17:40, 27 January 2017 (UTC))
(There should be four MOs for each of the TSs, formed by the four MOs in total of the reactants Tam10 (talk) 17:40, 27 January 2017 (UTC))
As shown by the MOs of the transition states, both the HOMO and LUMOs have a plane of symmetry, therefore were formed from the symmetrical orbitals of the reactants, i.e. the HOMO of 1,3-dioxole and the LUMO of cyclohexadiene. This is an inverse electron demand reaction in which the dieneophile is the lower energy component and donates electron density into the empty LUMO of the diene. This is known to occur when there is an electron withdrawing group on the diene (i.e. two electronegative oxygen atoms in this case) or an electron donating group on the dieneophile.[4] These characteristics mean that the dieneophile HOMO and diene LUMO are now the closest energy orbitals of the same symmetry which can interact.
Reaction Barrier Energies
The energies of the of the reactants, transition states and products were found by viewing the text files of the .log files. These energies are tabulated below in Table 2:
| Reactants | Endo TS | Exo TS | Endo Product | Exo Product |
|---|---|---|---|---|
| 195.49 | 362.16 | 364.69 | 99.25 | 99.70 |
These energies can be used to find the reaction barriers and reaction energies. The enthalpy change for the reaction is given by the difference between the reactants and products. For the endo reaction it is -96.21 kJ/mol, and for the exo reaction it is -95.79 kJ/mol. The endo reaction is slightly more exothermic than the exo and the product is slightly lower in energy, which means that the endo product is thermodynamically more stable (by a small amount).
The reaction barriers can be found by finding the difference between the reactants and the transition states, which for the endo product is 166.67 kJ/mol and for the exo product is 169.2 kJ/mol. The endo product has a lower reaction barrier since the transition state is lower in energy. The reasons for this are discussed in the next part of this section.
Secondary Orbital Interactions
Secondary Orbital Interactions are interactions between non-bonding orbitals, which can have a stabilising effect on the system and lower its overall energy. In the HOMO of the endo product in the reaction between 1,3-dioxole and cyclohexadiene there are secondary orbital interactions between the oxygen atoms on 1,3-dioxole and the pi orbitals at the back of the diene which lower the energy in the transition state[5]. This can occur because the orbitals have the correct orientation in the endo form, whereas in the exo form the oxygen atoms are not near the back of the diene and there are only primary orbital interactions in the exo product. These differences are illustrated in the TS HOMOs shown below:
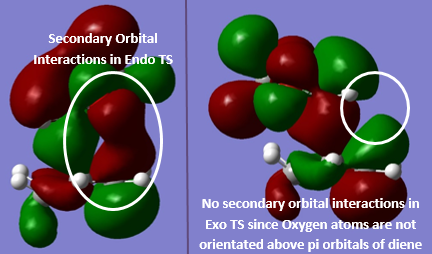
Nf710 (talk) 10:40, 30 January 2017 (UTC) Your theory was good in this section, good understanding and valid arguments however your energies were slightly out , but you have still come to the correct conclusion.
Exercise 3: Diels-Alder vs. Chelotropic
Xylylene and Sulfur Dioxide can undergo a variety of cycloaddition reactions to give different products. Xylylene, an unstable molecule, acts as the diene, and SO2 as the dieneophile. The reaction can proceed via an endo- or exo- Diels-Alder or via a Chelotropic Mechanism. These three possible outcomes are shown below in Scheme 2:

IRC Calculations
In this exercise, the products, TSs and reactants were optimised at the PM6 level using Gaussview and IRC calculations were carried out for each pathway. These IRCs are shown below:



Energy Profiles
The activation energies and reaction energies could be calculated (in kJ/mol) and used to draw reaction profiles of each of these reactions. These are shown below.


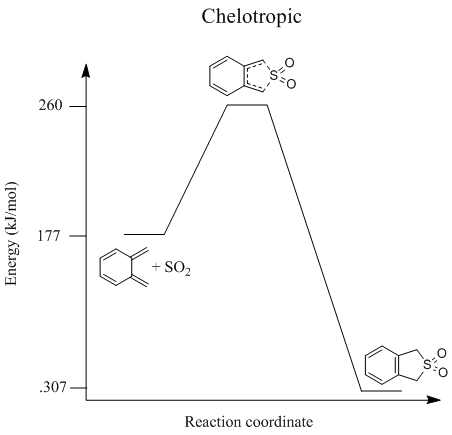
(These diagrams are easier to compare when on the same axis and normalised - it's difficult to accurately retrieve the activation and reaction energies from this Tam10 (talk) 17:44, 27 January 2017 (UTC))
This shows that the preferred reaction route (provided the reaction is irreversible) is the endo Diels-Alder reaction, since reaction has the lowest activation energy (this is the kinetic product). The exo Diels-Alder reaction produces product which is thermodynamically more stable than the endo, however the product of the chelotropic reaction is lowest in energy and therefore the most thermodynamically stable.
Throughout the course of the reaction, it can be seen that the six-membered ring of xylylene becomes aromatic.
References
- ↑ PAC, 1994, 66, 1077 (Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994)) pg 1174
- ↑ Carbon—Carbon Bond Distances. The Electron Diffraction Investigation of Ethane, Propane, Isobutane, Neopentane, Cyclopropane, Cyclopentane, Cyclohexane, Allene, Ethylene, Isobutene, Tetramethylethylene, Mesitylene, and Hexamethylbenzene. Revised Values of Covalent Radii Linus Pauling and L. O. Brockway 1937 59 (7), 1223-1236 DOI: 10.1021/ja01286a021
- ↑ A. Bondi J. Phys. Chem., 1964, 68 (3), pp 441–451 DOI: 10.1021/j100785a001 Publication Date: March 1964
- ↑ Juhl, K. and Jørgensen, K. A. (2003), The First Organocatalytic Enantioselective Inverse-Electron-Demand Hetero-Diels–Alder Reaction. Angewandte Chemie International Edition, 42: 1498–1501. doi:10.1002/anie.200250652
- ↑ Tetrahedron Volume 39, Issue 13, 1983, Pages 2095-2135 doi:10.1016/S0040-4020(01)91928-3
