Rep:Mod:cl308
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Author - Christopher Lloyd-Davies
Introduction
Molecular mechanics (MM) is a valuable technique that can be used to calculate the energy and properties of molecules[1]. The method relies on optimising the molecular geometry to give an energy minimum and in so doing provides the final energy in terms of bond length, angle strain, steric effects and van der Waals contributions. The advantage of this technique is that it provides an accurate answer in a short time-frame whilst it avoids solving the Schrödinger equation to obtain the exact energy. The method by which molecular mechanics works is by calculating independent terms that describe the respective bond property[2]:
- Sum of all diatomic bond stretches
- Sum of all triatomic bond angle deformations
- Sum of all tetra-atomic bond torsions
- Sum of all non-bonded Van der Waals attractions and repulsions
- Sum of all electrostatic attractions of individual bond dipoles
These functions are facile to solve using experimentally derived parameters. The method relies on experimentally derived constraints and the equations used are based upon simple diatomic cases, which leads to a breakdown in the method when modelling aromatic or non-classical systems.
The Hydrogenation of Cyclopentadiene Dimer
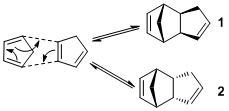
Cyclopentadiene will dimerise via a [4+2] Diels-Alder cycloaddition mechanism[3] (Figure 1).
XML error: Mismatched tag at line 11
The resulting reaction can form two possible configurational isomers but at room temperature the endo dimer is preferred over the exo dimer. The reason for this preference is a kinetic effect, specifically it concerns a secondary orbital effect. In the endo transition state orbitals that are not involved with bond formation are capable of interacting with and stabilising the entire transition state.
After creating models of both possible products (1) and (2), an MM2 force field was applied using ChemBio3D to find their respective optimised structure's energy minimum. This was done by considering all the relevant contributing forces acting on the molecule to find the conformation that provided the overall energy minimum and its associated value (Table 1.1)
| Relative Energies of Cyclopentadiene Dimers/kJmol-1 | ||||||||
| molecule | stretch | bend | stretch-bend | torsion | non-1,4 Van der Waals | 1,4 Van der Waals | Dipole-Dipole | Total |
| 1 | 5.36 | 86.19 | -3.51 | 32.01 | -5.94 | 17.70 | 1.59 | 133.39 |
| 2 | 5.27 | 87.24 | -3.51 | 39.79 | -6.44 | 18.03 | 1.88 | 142.26 |
The results from Table 1.1 show that the exo product (1) is thermodynamically more stable than the endo product (2). This observed thermodynamic instability can be largely explained by the increase in torsional strain in the endo product, which can be speculated to arise from the carbon bridge and the five-membered ring interacting (actual analysis is beyond the capabilities of ChemBio3D). In this way, the thermodynamic product is (1) whilst the kinetic product is (2)[4].
However, it is found that the reaction produces solely the less thermodynamically stable, endo product. This can be justified by the reaction being under kinetic control because of, as previously discussed, the secondary orbital interaction that arises from the endo transition state has a stabilising effect. It should also be noted that both endo and exo transition states exhibit a primary orbital interactions. The preference for endo-selectivity as shown by the literature, can be supported by the Woodward-Hoffmann selection rule[5][6].
If the endo dimer were now to be partially hydrogenated there are two possible sites of hydrogenation: either on the pentacyclic ring to give the dihydro derivative (3), or on the hexacyclic ring to give the dihydro derivative (4), (Figure 2). There is also the possibility of full hydrogenation to the tetrahydro derivative that arises after excessive hydrogenation[7].

XML error: Mismatched tag at line 11
In a similar way, the structures of (3) and (4) were optimised to minimise the energy of the molecules using ChemBio3D and the resulting data was compared in Table 1.2.
| Relative Energies of dihydro derivatives/kJmol-1 | ||||||||
| molecule | stretch | bend | stretch-bend | torsion | non-1,4 Van der Waals | 1,4 Van der Waals | Dipole-dipole | Total |
| 3 | 5.34 | 82.86 | -3.48 | 45.47 | -5.11 | 23.59 | 0.68 | 149.35 |
| 4 | 4.58 | 60.70 | -2.29 | 52.33 | -4.42 | 18.91 | 0.59 | 130.38 |
The results of Table 1.2 show that (4) is more thermodynamically stable than (3), which can be considered the kinetic product. The largest discrepancy between each contributing energy term is that of the bending, which shows a large difference of 22.16kJmol-1. This can be rationalised by examining the sp2 carbon angles. In the more thermodynamically stable regioisomer (4) the sp2 angles were 112o and 113o (Figure 4), whilst the angles in (3) were 108o (Figure 3). These angles show that the sp2 angles in (3) were further from the ideal 120o, making the molecule more strained and thus less stable.
 |
 |
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
This experiment concerns two reactions involving nucleophilic attack on C4 of the pyridine ring of a NAD derivative and specifically the regio- and stereo- selectivity observed. In the first case an optically active derivative of prolinol (5) can be alkylated using a grignard reagent to form (6)[8]. The observed high specificity with which the product forms can be explained using data from molecular mechanics modelling. The mechanism is suggested in Figure 5.
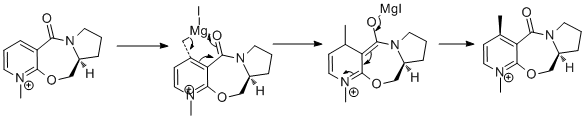
The regioselectivity of the addition to C4 can be understood through the interaction between the oxygen of the amide carbonyl and the attacking grignard reagent. It has been suggested by Schultz et al[8] that the incoming magnesium of the grignard reagent, which is electropositive coordinates with the electronegative oxygen; this in turn delivers the methyl group to C4.
On attempting to perform an energy minimisation using the MM2 model in ChemBio3D on the prolinol derivative with the grignard reagent coordinated, as suggested, an error message is obtained. The reason for this is that the magnesium atom has not had its parameters defined and hence an error occurs. It is not supported because the MM2 model has a set of parameters defined for different element types but magnesium isn’t included in these. This limitation of the model could be overcome by using a MOPAC molecular orbital interface.
The energy was recorded using MMFF94 model and recorded in Table 1.3. It was noted that there is very limited flexibility within the hepta-membered ring, hinting that the molecule would be stabilised by the orientation of the carbonyl group. The positive nature of the dihedral value (12.72o) signals that the carbonyl group is above the ring. This supports the idea that the grignard reagent will coordinate to the oxygen via the lone pair resulting in the observed product's stereochemistry.
| MMFF94 calculated total energies for molecules 5 and 6/kJmol-1 | |||
| Molecule | Total Energy (kJmol-1) | Dihedral Angle | |
| 5 | 240.26 | 12.72 o | |
| 6 | 411.13 | -37.3 o | |

The next reaction examined was a reaction of N-methyl quinolinium salt (6) with aniline[9]. Using a similar method as described above, using the model MMFF94, the minimum energy conformation was found. The stereochemistry observed within this reaction can be explained by the presence, and close proximity of, electron-rich nitrogen on the aniline and the carbonyl oxygen whose lone pairs will repel. The repulsion of these atoms, combined with the orientation of the carbonyl group will determine the orientation of the aniline with respect to the ring (Figure 6). In this way it was observed that the lowest energy conformation arose when the dihedral angle (Table 1.3) across the carbonyl was negative, signalling that it was beneath the ring and thus enabling the aniline to attack from the top surface giving the observed product[10].
A better approach to modelling would be to consider the electronics of the reaction which MM2 cannot do. Thinking about orbital interaction in reactions and molecules gives a greater understanding of what is actually happening. To get to grips with the electronic properties of a reaction leads to an overall improved comprehension, which cannot be gleaned from using molecular mechanics. In this way, MOPAC/PM6 interface would be a good option.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol

The synthesis of taxol, an important anti-cancer drug, proceeds via one of two intermediates (Figure 7) with the carbonyl group pointing up (9) or with the carbonyl group pointing down (10)[11]. These can be considered atropisomers, which are stereoisomers that are hindered by steric strain and hence are limited in their ability to rotate about single bonds thus preventing interconversion between isomers. The rotation of the bonds either side of the carbonyl group is limited due to steric strain as the carbonyl group rotates. It was also seen that the atropisomers isomerised to a single carbonyl isomer, which was noted to have high alkene stability. To appreciate the outcome of an addition to the carbonyl group each atropisomer is modelled and the energy is minimised. It is thus possible infer which atropisomer is the major form. A reason behind the rigidity of the intermediate is the existence of a bridgehead alkene that gives a rigid structure for the rest of the molecule.
The different conformations of the cyclohexane were manually altered to find the energy minimum when modelled using MM2 in ChemBio3D. The total energy was then compared with the energy obtained from a MMFF94 model (Table 1.4). The energies found for both molecules were lowest when the molecules where in the chair conformation. Higher energy conformers were investigated with twist-boat conformers on both the "up" and "down" carbonyl atropisomers being found to be far less stable. There was also another chair conformer for the "down" carbonyl atropisomer, which was higher in energy than the "up" carbonyl atropisomer.
| Relative Energies of Taxol Intermediates/kJmol-1 | |||||||||
| molecule | stretch | bend | stretch-bend | torsion | non-1,4 Van der Waals | 1,4 Van der Waals | Dipole-dipole | Total (MM2) | Total (MMFF94) |
| 9 | 11.21 | 66.26 | 1.65 | 76.20 | -4.37 | 52.92 | 0.62 | 204.49 | 295.32 |
| 10 | 10.66 | 41.65 | 1.16 | 81.11 | -6.28 | 52.74 | -0.78 | 180.26 | 255.98 |
On inspection of Table 1.4 it is clear that the atropisomer with the carbonyl "down" is far more stable (by 24.23kJmol-1 using MM2 result). The difference in energy can be largely attributed to the bend energy contribution, which in (9) is 14.61kJmol-1 larger than in (10). Once the angles around the sp2 carbonyl carbon, ideally 120o, had been examined it was clear that the greater deviation in (9) compared to (10) caused the observed difference in energy (Figure 8 & 9).
 |
 |
The reported decrease in double bond reactivity within the molecule can be attributed to the large size of the ring. Initially, it would have been fair to assume, as discussed by Bredt's rule[12], that the bridgehead double bond would have shown increased reactivity. However, the rule can be neglected due to the decamembered carbon ring. In fact, the size of the ring is what causes the increased stability with the double bond. The large ring causes increased strain on sp3 carbons since the large ring requires angles far larger than the desired 109o for an sp3. In this way, the large angles forced by the ring cause less strain on sp2 carbons since their ideal angle is 120o. As a result it is beneficial to have some sp2 carbons in large rings to alleviate the bending strain.
This can be investigated by examining the hydrogenation product's energy contributions. Comparing the results of Table 1.4 and the minimum energy (MM2 Model) obtained for the hydrogenated product (11) XML error: Mismatched tag at line 7 (216.87kJmol-1) it is clear that there has been a drastic increase in energy. This can be justified by looking at the angles of the newly hydrogenated carbons, whose angles are far closer to the ideal of an sp2 carbon rather than their preferred sp3 geometry. Despite being a simple comparison it is clear that if such small substituents as hydrogen atoms can cause such a large energy destabilisation larger groups would have a far greater effect.
Molecule (10), despite bond enthalpy considerations, can be considered a hyperstable alkene[13] since the energy of the alkene subtracted from the energy of the corresponding alkane (11) gives a negative number (-39.11kJmol-1), known as the olefin strain energy - typically negative for hyperstable alkenes.
Modelling Using Semi-empirical Molecular Orbital Theory
In completing the previous tasks several problems with molecular mechanics have arisen. The main issue was the method's inability to take into consideration electronic behaviour. To do so, semi-empirical methods can be used to give greater insight into the propensity of electron donation or acceptance at a given location on a molecule.
It has been reported that (12) (Figure 10) is found to react regioselectively with dichlorocarbene[14]. The addition of dichlorocarbene across the syn alkene has been shown to produce the mono-adduct endo-product (72%) and the di-adduct (23%), but yet no mono anti alkene was seen (Figure 11).
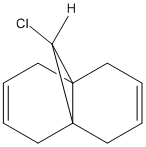
XML error: Mismatched tag at line 7
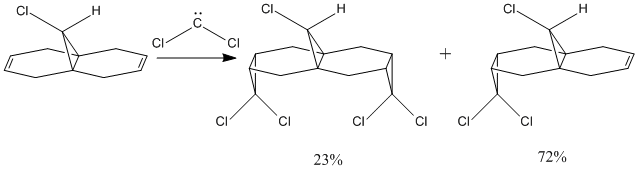
In this way the molecular orbitals of (12) were investigated to give insight into the observed regioselectivity. Initially (12) was drawn in ChemBio3D and the energy was minimised using the MM2 model (Table 1.5). The energy of formation (82.59kJmol-1) was calculated using the MOPAC/PM6 technique.
| Relative Energies of 9-Chloro-1,4,5,8-tetrahydro-4a,8a-methanoaphthalene/kJmol-1 | |||||||||
| molecule | stretch | bend | stretch-bend | torsion | non-1,4 Van der Waals | 1,4 Van der Waals | Dipole-Dipole | Total | |
| 12 | 2.59 | 19.81 | 0.17 | 32.05 | -4.46 | 24.24 | 0.47 | 74.87 | |
The molecular orbitals were then calculated and drawn using the MOPAC/PM6 method [15].
 |
 |
 |
 |
 |
The reaction in Figure 11 with dichlorocarbene is an electrophile and thus will occur at the most electron rich alkene. On inspection of the HOMO it is clear that the most electron density is around the double bond (syn alkene) beneath the chlorine atom. In addition to this, there is also a stabilising anti-periplanar interaction between the anti alkene π orbital and the C-Cl σ* orbital. This interaction can be seen as the HOMO-1 and the LUMO+1 overlapping to stabilise the alkene. From this it is clear that the anti alkene is less reactive since it has been stabilised relative to the syn alkene, hence explaining the reaction's regioselectivity.
| IR vibrations(cm-1) of derivatives of 12 | ||||
| molecule | C-Cl stretch | C=C(exo) stretch | C=C(endo) stretch | |
| 12 XML error: Mismatched tag at line 7 | 770.86 | 1757.37 | 1737.15 | |
| monoalkene | 779.74 | 1753.78 | n/a | |
| OH substituted XML error: Mismatched tag at line 7 | 765.36 | 1753.01 | 1757.73 | |
| BH2 substituted XML error: Mismatched tag at line 7 | 759.08 | 1756.51 | 1657.14 | |
| SiH3 substituted XML error: Mismatched tag at line 7 | 763.82 | 1756.32 | 1690.27 | |
| CN substituted XML error: Mismatched tag at line 7 | 765.68 | 1756.56 | 1706.44 | |
On examining the data in Table 1.6 it is found that the stretch values for the C-Cl bond correspond well to the literature value. The values obtained for the C=C double bond stretches are marginally higher than the literature values, which signals that the bonds are more stable. The extra stability observed arises from the rigid bicyclic structure and the fact that chlorine atom can donate electron density to the π bonds. From the table the data suggests that the exo bond(syn C=C) appears stronger than the endo bond(anti C=C) due to it having a higher wavenumber, but this contracdicts the aforementioned argument. The contradiction can be explained by the fact that the π electrons are being examined. Some of the electron density of the π electrons in the anti alkene will be withdrawn towards to the C-Cl bond. This reduces the π electron density - thus increasing the σ character of the bond. π electrons are found to be less stable than σ electrons and so the bond exhibits increased stability.
In the case of the monoalkene derivative that has had the anti hydrogenated it is found that the C-Cl bond strength increases, this is because of the lack of interaction between π electrons of the anti double bond and the σ* orbital of the C-Cl bond, which if present would have weakened the C-Cl bond.
Further conclusions from the different substituents can be drawn. When OH is the substituent there is an decrease in wavenumber of the C-Cl stretch to 765.36cm-1. This is because OH is an electron donating group, due to the lone pairs, and it is thought that an increase in electron density in the exo (syn) double bond would occur. This would lead to an increase of electron density in the C-Cl σ* orbital which would weaken the bond, causing the decrease in wavenumber. The increase in electron density in the C-Cl σ* orbital would cause a greater interaction between π electrons of the anti double bond and the σ* orbital of the C-Cl bond, causing a stronger anti bond.
BH2 substituted group has a drastic effect on the wavenumber of the endo double bond because of it acting like a lewis acid, accepting electrons from the π bond into its low lying unoccupied p-orbital which would weaken the alkene bond. The same effect is observed in the silyl group but to a lesser extent since the silicon's d-orbitals are higher in energy and worse at accepting electron density.
The cyano group withdraws electron density and hence reduces the wavenumber.
Structure based Mini project using DFT-based Molecular orbital methods
Assigning Regioisomers in "Click Chemistry"
Introduction
"Click Chemistry" concerns reactions that are fast and involve small starting materials that maximise atom economy with high overall specificity. In this case,a triazole will be made from catalysed 1,3-dipolar cycloadditions,using different catalysts (Copper(II) or Ruthenium (II)) results in different isomers being made (Figure 17)[16]. The formation of different isomers can be explained by the different mechanisms involved with the different catalysts. In the case of the the Ruthenium (II) catalyst the azide undergoes nucleophilic oxidative addition, to which the alkyne is added. A hexa-membered species involving the Ruthenium is made before the triazole is reductively eliminated. In the case of the Copper (II) catalsyt the alkyne is deprotonated before adding to the copper, which is oxidised to copper(I). Again, a hexa-membered ring is formed involving the copper and then reductive elimination occurs eliminating the triazole.
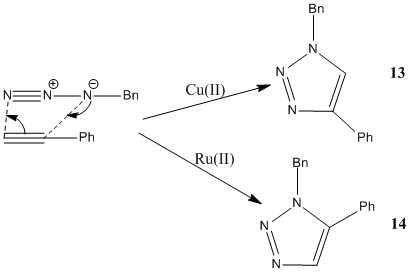
XML error: Mismatched tag at line 11
Initially, the MM2 model was run to minimise the energy for both products, it was noted that the MM2 still had some parameters undefined. It was clear that (13) had a lower energy compared to (14) (44.56kJmol-1 and 72.05kJmol-1). These structures were then exported to gaussview before being run on SCAN to minimise the structures once again. The NMR and IR computed data was then calculated.
Calculated NMR
 |
 |
The data corresponds to the relevant numbered carbon (Figure 20)
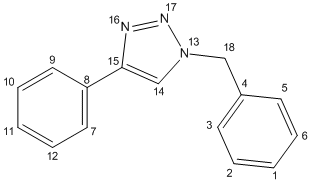
| Calculated NMR data - 13 | ||
| calculated δ/ppm | atom | |
| 51.7 | 8 | |
| 63.4 | 4 | |
| 68.9 | 8 | |
| 71.0 | 6 | |
| 71.4 | 10 | |
| 71.6 | 12 | |
| 71.8 | 1 | |
| 72.0 | 2 | |
| 72.3 | 3 | |
| 72.5 | 5 | |
| 74.7 | 9 | |
| 75.3 | 7 | |
| 79.6 | 14 | |
| 141.7 | 18 | |
The data below corresponds to the relevant numbered carbon (Figure 21)
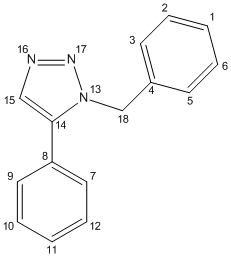
| Calculated NMR data - 14 | |||
| atom | calculated δ/ppm | lit δ[17]/ppm | |
| 14 | 59.9 | 51.85 | |
| 4 | 62.5 | 126.93 | |
| 15 | 68.0 | 127.22 | |
| 9 or 7 | 70.4 | 128.22 | |
| 11 | 70.7 | 128.22 | |
| 10 or 12 | 71.0 | multiplet ~129 | |
| 10 or 12 | 71.1 | multiplet ~129 | |
| 2 or 6 | 71.4 | multiplet ~129 | |
| 8 | 71.5 | multiplet ~129 | |
| 2 or 6 | 71.9 | 129.64 | |
| 3 or 5 | 72.1 | 133.26 | |
| 1 | 72.9 | 133.34 | |
| 9 or 7 | 73.2 | 135.66 | |
| 18 | 144.3 | 138.26 | |
From this data it is quite hard to decide the key distinguishing features of the spectra. However, it clear that from examining the spectra it would be possible to distinguish between the isomers. Key peaks are the first peak for the connecting phenyl carbon (there is a large difference between the isomers for the shift and so could be used) and also the last peak for the benzyl carbon that links the triazole and the phenyl ring.
The above spectra were obtained from gaussview using "# mpw1pw91/6-31(d,p) NMR scrf(cpcm,solvent=chloroform)" as the line of the gaussview input file program. It was clear that these values were askew from the obtained literature values, which might be explained by the calculated values failing to account for the degeneracy of the system since it has treated every carbon as distinguishable. It is hard to ascertain the reason for the observed deviations since the structures were minimised using the MM2 model, then MOPAC/PM6, and it was this structure that was then minimised in gaussian and finally had its NMR calculated.
There would be more analysis but I have run out of time for the deadline.
Conclusions
Further investigations that could have been done would have been to calculate an IR for both isomers. Unfortunately, because the molecules are almost identical in terms of types of bonds present it would be expected that the IR spectra to be very similar - if not practically indistinguishable. A far more conclusive and rewarding analysis is the 13C, which has already been done. A problem with the calculated data of the NMR is that quantum mechanics is unable to consider the random motion of the isomer. Another type of spectroscopy that might be useful is nuclear overhauser effect spectroscopy, which can be used to measure the distance between two hydrogens on the isomer. This would distinguish between the isomers.
References
- ↑ N.Allinger, J. Am. Chem. Soc., 1977, 99(25), pp 8127–8134:DOI:10.1021/ja00467a001
- ↑ Molecular mechanics Chemistry Wiki page Mechanics
- ↑ J. A. Norton, Chem. Rev., 1942, 31 (2), 319–523 DOI:10.1021/cr60099a003
- ↑ W. C. Herndon, C. R. Grayson, J. M. Manion, J. Org. Chem., 32 (3), 1967, 526–529 DOI:10.1021/jo01278a003
- ↑ R.Jarret, J.New, R.Hurley & L.Gillooly J. Am. Chem. Soc. 2001, 78 pp1262:DOI:10.1021/ed078p1262
- ↑ Stereochemistry of Electrocyclic Reactions:DOI:10.1021/ja01080a054
- ↑ Skala, D. Hanika, J. Petroleum and Coal. 2003, 45(3-4), 105-108[1]
- ↑ 8.0 8.1 Shultz, A. et al. J. Org. Chemistry, 1986, 51, 838[2]
- ↑ Leleu, Stephane; Papamicael, Cyril; Marsais, Francis; Dupas, Georges; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928. DOI:10.1016/j.tetasy.2004.11.004
- ↑ Chem. of Hetrocyclic Compounds, 1994, 30(11-12), pp 1331–1385:DOI:10.1007/BF01172864
- ↑ Elmore, S. Paquette, L. Tetrahedron Letters, 1991, 32(3), 319-222. [3]
- ↑ Bredt, J. Annalen der Chemie, 1924, 437: 1–9 Error: Bad DOI specified!
- ↑ Camps, P. et al. Tetrahedron, 1997, 53(28), 9727-9734. [4]
- ↑ Halton, B. et al. J. Chem. Soc., Perkin Trans. 2, 1992, 4, 447-448.
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
- ↑ Hartmuth C. Kolb, M. G. Finn, K. Barry Sharpless, Angew. Chem. Int. Ed. 2001, 40, 2004, pp.2021 DOI:<2004::AID-ANIE2004>3.0.CO;2-5 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
- ↑ Ionic Polymer Supported Copper(I): A Reusable Catalyst for Huisgen's 1,3-Dipolar Cycloaddition, Uthaiwan Sirion, Yu Jin Bae, Byoung Se Lee, Dae Yoon Chi, Synlett 2008 2326-2330 DOI:10.1055/s-2008-1078245
