Rep:Mod:cjmodule1
Module1
Exercise 1: Dimerisation of Cyclopentadiene and Hydrogenation of Dicyclpentadiene
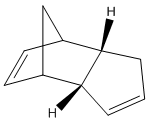
A) Dimerisation of Cyclopentadiene
- Calculation of the energy of the Exo and Endo dimer by using the Molecular Mechanics Program (MM2)
Comments:
The total energy of exo dimer is approximately 2 kcal/mol lower than endo dimer is, which the higher energy is mainly caused by the torsional strain of endo dimer, this difference of total energy indicates the exo dimer is thermodynamically more stable. However, both exo and endo dimers have a structure of bridgehead and C=C double bond, therefore, the bending factor of these molecules become the major contribution to the total energy.
But in reality, the endo dimer 2 is formed instead of the exo dimer 1, which has higher thermodynamic energy (less favour) than exo. This is due to the ignorance of kinetic energy in MM2 programme. The observation implies that the EA (activation energy) of endo dimer is significantly lower than exo dimer, and it even overcomes the disfavoured thermodynamic factor, and hence, the dimerisation of cyclopendiene need to be under kinetic control.
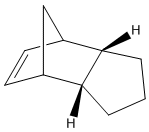
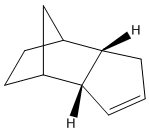
B) Hydrogenation of Dicyclpentadiene
- Determination of thermodynamic stability of hydrogenation product from endo dimer 2 by MM2
Comments:
The total energy of hydrogenation product 4 is approximately 5 kcal/mol lower than product 3, which the higher energy is mainly caused by the bending destabilisation and 1,4-VDW interaction of product 3, this difference of total energy indicates the product 4 is thermodynamically more stable.
The product 4 would be the main product if the hydrogenation was carried out under thermodynamic control, this is due to relieve of bending destabilisation. Although both products have bridgehead and C=C bond, but product 3 has its alkene group much nearer to the bridgehead than product 4, and this causes the extra bending destabilisation of 3. As explained in part (A), the data from MM2 programme only involves thermodynamic factor, and hence, there is no enough information to decide whether the kinetic factor will overcome the thermodynamic factor and to favour hydrogenation product 3. And also, in order to determine which hydrogenation product forms primarily, the intermediate transition state geometry would be required.
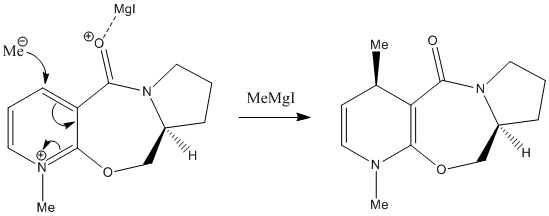
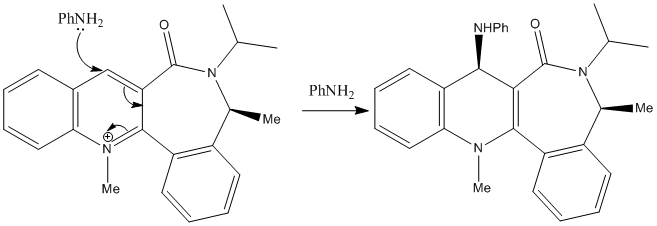
Exercise 2: Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
(A) Reaction 1
Mechanism
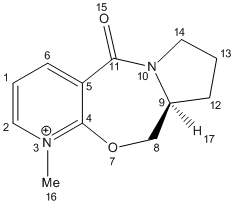
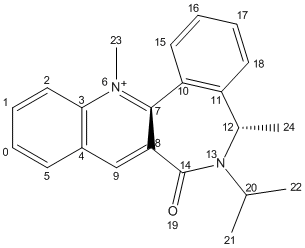
1) Optimisation of Molecule 5
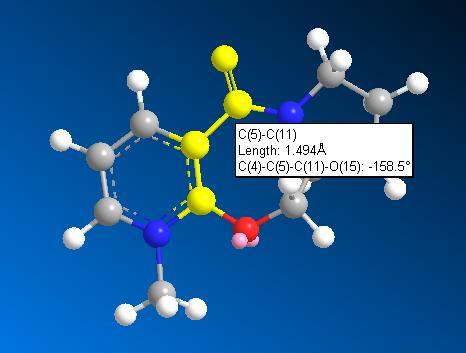
Molecule 5 was predicted to have a total energy of 26.35kcal/mol by using MM2. In order to minimise the energy of the molecule, an optimisation procedure was carried out by changing the spatial arrangement of the 7-membered ring (atom 7 to 11) and repeating the total energies by MM2. As the planar aromatic system already has its lowest energy geometry fixed, changing the spatial arrangement of substituent and atoms on aromatic system would make no difference to the total energy. And also, there was not any change in total energy by changing atoms 12 to 14, which means they have no significant influence on this. However, the total energy deceased to 25.72 kcal/mol by stretching atom 8 and 11.
Higher energy conformation was given out by changing spatial arrangement of the Nitrogen atom 10 and Oxygen atom 15.
Therefore, the optimised structure of molecule 5 is: (in Jmol structure)
With dihedral angle of atoms 4-5-10-11 158.5o.
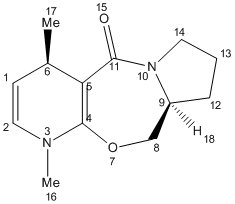
2) Optimisation of Molecule 6
Molecule 5 was predicted to have a total energy of 28.85 kcal/mol by using MM2, which is approximately 5kcal/mol higher in total energy, and hence thermodynamically less stable than molecule 5. This may be caused by introducing the methyl group 17 and so increasing the strain of molecule 6, and also, the removal of aromaticity. And hence, the reaction is under kinetic control.
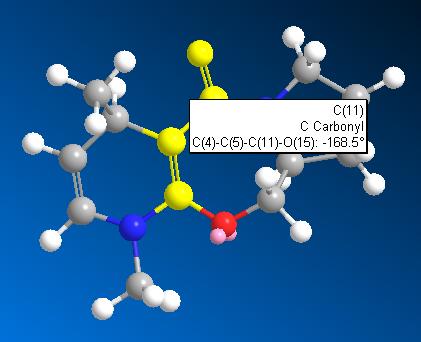
Again, by applying the optimisation procedure, as molecule 5 and 6 are very similar to each other, atom 8 and 11 were moved in a same manner to molecule 5, and this optimised the total energy to 28.34 kcal/mol. (as shown below)
With dihedral angle of atoms 4-5-10-11 168.5o.
3) Conclusion on Behaviour of Nuclecophillic Addition
The positive charge created by complexation of the MgI group on carbonyl oxygen stabilised the 6-membered intermediate transition state, and also determined which face of carbonyl the nucleophile will attack (same face of the C=O pointing towards). As shown in part 2) and considering the dihedral angle, in case of carbonyl group pointing outwards the plane of paper, the Me- required to attack from the top face in order to achieve the stereochemistry. The molecule 6 is relatively thermodynamically unstable due to electronic repulsion from same orientation of methyl and carbonyl groups, and so, the activation energy decreased and 6-membered intermediate transition state should be under kinetic control.
(B) Reaction 2
Mechanism
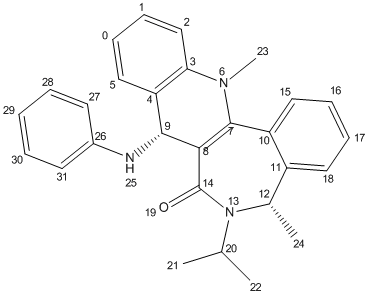
1) Optimisation of Molecule 7
Molecule 7 was predicted to have a total energy of 14.8583 kcal/mol by using MM2. A lower energy conformation was optimised by stretching atom 14. Total energy decreased to 12.9658 kcal/mol.
Higher energy conformation was given out by changing spatial arrangement of isopropyl carbon atoms (20 to 22).
Therefore, the optimised structure of molecule 7 is:-
With dihedral angle of atoms 7-8-14-19 137.9o.
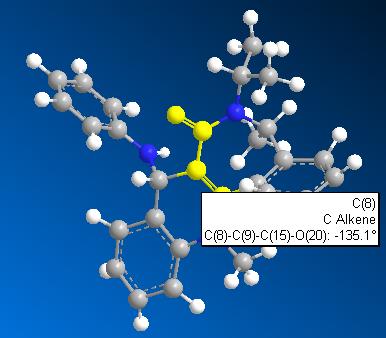
2) Optimisation of Molecule 8
As a result of stabilisation of molecule 7, the molecule 8 was predicted to have a total energy of -5.13 kcal/mol by using MM2. This significant lower in energy (approx. 20 kcal/mol difference) indicates the reaction is strongly controlled by thermodynamic factor. A lower energy conformation was optimised by stretching atom 14. Total energy decreased to -12.0332 kcal/mol.
Therefore, the optimised structure of molecule 8 is:-
With dihedral angle of atoms 7-8-14-19 135.1o.
3) Conclusion on Behaviour of Nuclecophillic Addition
As different from reaction (A), there is no complexation stabilisation to the carbonyl in this case, the nucleophile is very bulky and attacked from the opposite face from the carbonyl in order to optimise the steric hindrance. The stereoselectivity in this nucleophilic addition is majorly contributed by C3-Carbonyl bond, and so, the attacking of bulky NHPh- was controlled sterically.
(C) Reference
1. A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
2. Leleu, Stephane; Papamicael, Cyril; Marsais, Francis; Dupas, Georges; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928. DOI:10.1016/j.tetasy.2004.11.004
Exercise 3: Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
-The orientation of carbonyl addition is determinate by stability of reaction intermediate isomer. The MM2 programme is used to distinguish the energy of both isomers.
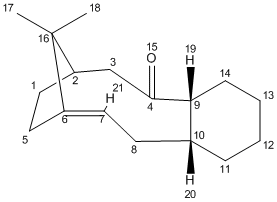
(A) Carbonyl Group Pointing Upwards - Isomer 10
 |
Stretch: 2.8214 |
| Bend: 16.4229 | |
| Stretch-Bend: 0.4517 | |
| Torsion: 21.3606 | |
| Non-1,4 VDW: -0.8070 | |
| 1,4 VDW: 14.0138 | |
| Dipole/Dipole: 0.1370 | |
| Total Energy: 53.4004 kcal/mol |
A total energy of 53.4004 kcal/mol was obtained from MM2 initially. A optimisation procedure was taken by changing the position of C(2) carbon and stretching out the C(4)=O(15) carbonyl group further apart from the ring system. As a result, a lowering total energy (mainly contributed by bending) was achieved by taking the carbonyl group was facing the same orientation as the bridgehead C(16) carbon. An optimised total energy of 52.3401 kcal/mol was obtained.
The cyclohexene part of isomer 10 adopts a twisted-boat conformation after optimisation.
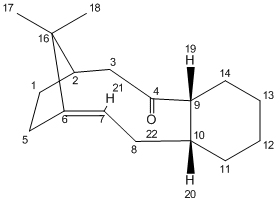
(B) Carbonyl Group Pointing Downwards - Isomer 11
 |
Stretch: 2.8103 |
| Bend: 16.4550 | |
| Stretch-Bend: 0.4564 | |
| Torsion: 21.4508 | |
| Non-1,4 VDW: -0.9572 | |
| 1,4 VDW: 14.0798 | |
| Dipole/Dipole: 0.1344 | |
| Total Energy: 54.4295 kcal/mol |
A total energy of 54.4295 kcal/mol was obtained from MM2 initially. Same as isomer 10, an optimisation procedure was taken by changing the position of C(2) carbon and stretching out the C(4)=O(15) carbonyl group further apart from the ring system. As a result, a lowering total energy (mainly contributed by bending) was achieved by taking the carbonyl group was facing the same orientation as the bridgehead C(16) carbon. For further optimisation, the cyclohexene ring was altered to have a chair-ish conformation with removing of all protons on it. An optimised total energy of 53.6736 kcal/mol and 50.3231 kcal/mol were obtained respectively.
The cyclohexene part of isomer 11 adopts a chair-ish conformation after optimisation.
(C) Comparison between isomer 10 and 11
Consider isomer 10, the cyclohexene ring is very rigid due to blocking by a bridged trans-cyclononenone at its central position, and so, a twisted boat conformation is adopted although it is thermodynamically unstable. Whereas in isomer 11, a chair-ish conformation is adopted due to non-bonded transannular interaction effect, which located at the centre of the cyclohexene ring. The total optimised energy of isomer 10 is approximately 1.01 kcal/mol less than isomer 11, and so the isomer 10 is more favoured than 11. However, due to the optimised total energy is fairly small, it is quite hard to tell which isomer will form exactly.
(D) Why does the alkene react slowly in both isomers?
The both alkene in these two isomers are defined as ‘hyperstable’ and they are relatively stable. The alkene react slowly because it located adjacent to a bridgehead functional group, which thermodynamically stabilised the molecule relatively to all the other positional isomers, and so this will be the only possible conformation when alkene can be involved in a ring larger than seven atoms as the Bredt’s Rules are not applied. Although the torsional strain in this form is much larger planar alkene, this is an alleviation when alkene taking part in a ring larger than seven.
(E) Reference
1. S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
2. J. G. Vinter and H. M. R. Hoffman, J. Am. Chem. Soc., 1974, 96, 5466 (DOI:10.1021/ja00824a025 DOI:10.1021/ja00824a025 ) and 95, 3051 for another nice example of atropisomerism.
3. J. Am. Chem. Soc., 1999, 121, 3226. DOI: 10.1021/ja990189i
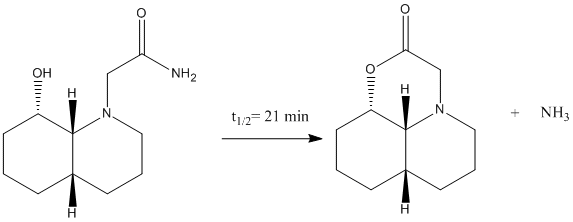
Exercise 4: How One Might Induce Room Temperature Hydrolysis of a Peptide
-In this exercise, by considering kinetic factors, explain the reaction why the half life of hydrolysis reaction 1 (isomer 13) is much faster than 2 (isomer 14).
(A) Rate of Reactions
Reaction Scheme 1 – Cis Decline (Isomer 13)
Reaction Scheme 2 – Trans Decline (Isomer 14)
The alcohol and amide functional groups are not under much steric compression although both cis- and trans-decline experience significant amount of strain. This explains why the reaction will not take a period of infinite time.
(B) Diastereoisomers
- For each E- and Z-isomers, there are two possible diastereoisomers. The major reactant will be the isomer which is more stable.
1) Z-isomer
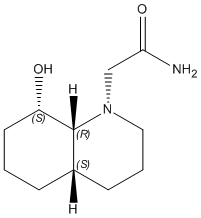 |
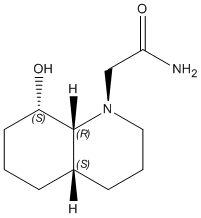 |
| Equatorial position of OH group | |
| Equatorial ethylamino:- | Axial ethylamino:- |
| Stretch: 1.5913 | Stretch: 1.5477 |
| Bend: 6.1513 | Bend: 8.0822 |
| Stretch-Bend: 0.6046 | Stretch-Bend: 0.6439 |
| Torsion: 8.6218 | Torsion: 13.2371 |
| Non-1,4 VDW: -5.2791 | Non-1,4 VDW: -8.0185 |
| 1,4 VDW: 9.3494 | 1,4 VDW: 10.5578 |
| Dipole/Dipole: -6.7971 | Dipole/Dipole: -4.9313 |
| Total Energy: 14.2421 kcal/mol | Total Energy: 19.1189 kcal/mol |
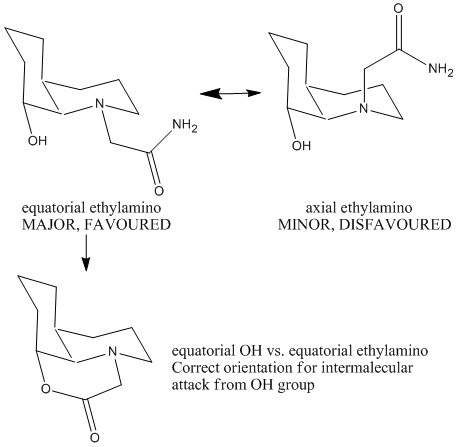
The optimisation energy of equatorial ethylamino isomer is 14.2421 kcal/mol, the structure is stabilised by intramolecular hydrogen bonding between the lone pair on OH group and the amide H. The optimisation energy of axial ethylamino isomer is 19.1189 kcal/mol, which is approximately 4.9 kcal/mol higher than equatorial one, this destabilisation is due to further apart between OH and amide H, ie. weaker H-bonding stabilisation, and also diaxial syn-conformation. The total energy difference agrees quite well with the literature value 5 kcal/mol. And hence, the lower energy equatorial ethylamido isomer is the major isomer of cis-decalin.
The Jmol for equatorial ethylamino cis-isomer:
2) E-isomer
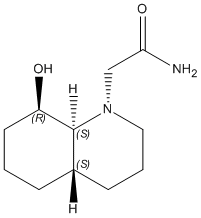 |
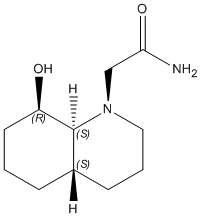 |
| Axial position of OH group | |
| Equatorial ethylamino:- | Axial ethylamino:- |
| Stretch: 1.4640 | Stretch: 1.6954 |
| Bend: 3.8110 | Bend: 5.4192 |
| Stretch-Bend: 0.5032 | Stretch-Bend: 0.6088 |
| Torsion: 7.7250 | Torsion: 8.8337 |
| Non-1,4 VDW: -7.1886 | Non-1,4 VDW: -5.2690 |
| 1,4 VDW: 9.9157 | 1,4 VDW: 9.5625 |
| Dipole/Dipole: -6.5536 | Dipole/Dipole: -6.8288 |
| Total Energy: 9.6766 kcal/mol | Total Energy: 13.0217 kcal/mol |
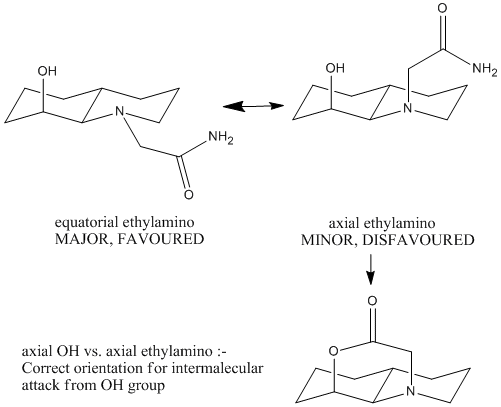
The optimisation energy of equatorial ethylamino isomer is 9.6766 kcal/mol, the structure is stabilised by intramolecular hydrogen bonding between the OH group and the carbonyl oxygen, and also the chair-chair conformation. The optimisation energy of axial ethylamino isomer is 13.0217 kcal/mol, which is approximately 3.3 kcal/mol higher than equatorial one, this destabilisation is due to further apart between OH and amide H, ie. weaker H-bonding stabilisation, and also diaxial syn-conformation. The total energy difference agrees quite well with the literature value 3.1 kcal/mol. And hence, the lower energy equatorial ethylamido isomer is the major isomer of trans-decalin.
The Jmol for equatorial ethylamino trans-isomer :
3) Comparison of 4 Diastereomers
| Cis – Decalin | Major Isomer | 14.2421 kcal/mol |
| Major Isomer | 19.1189 kcal/mol | |
| Trans – Decalin | Major Isomer | 9.6766 kcal/mol |
| Major Isomer | 13.0217 kcal/mol |
Trans-Decalin has greater energy for both major and minor isomers compare to cis-decalin, this indicates that trans-decalin isomers have less torsional strain than cis-decalin.
4) Reaction scheme for cis- and trans- declins in Chair-Chair conformation
The OH group and amide H need to be closed enough in order to let intramolecular nucleophilc attacking to happen.
For cis- decalin:-
The correct orientation for nucleophilc attacking is the same one as the major isomer for cis-decalin, ie. equatorial OH vs. equatorial ethylamino.
Whereas for trans- decalin:-
The correct orientation for nucleophilc attacking is NOT the same one as the major isomer for cis-decalin, but the minor isomer! ie. axial OH vs. axial ethylamino. Therefore, in order to let the intramolecular nucleophilc attack to happen, the favoured major isomer must be converted into the disfavoured minor isomer, this conversion requires energy and hence built up higher activation energy barrier.
And hence, the trans-decalin reacts much slower than cis- does.
(C) Reference
1. M. Fernandes, F. Fache, M. Rosen, P.-L. Nguyen, and D. E. Hansen, 'Rapid Cleavage of Unactivated, Unstrained Amide Bonds at Neutral pH', J. Org. Chem., 2008, 73, 6413–6416 ASAP: DOI:10.1021/jo800706y
Exercise 5: Modelling Using Semi-empirical Molecular Orbital Theory - Regioselective Addition of Dichlorocarbene
-Previously, the strengths and weaknesses of a purely mechanical molecular model were illustrated. The limitation of Molecular Mechanics system was shown. In this section, the regioelective addition of dichlorocarbene is illustrated by MM2 in a quantum based methodology, and also the spectroscopic properties of the molecule.
(A) Molecule 12
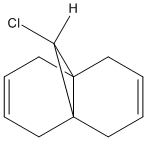 |
Stretch: 0.6273 |
| Bend: 4.6716 | |
| Stretch-Bend: 0.0396 | |
| Torsion: 7.7055 | |
| Non-1,4 VDW: -1.0669 | |
| 1,4 VDW: 5.8088 | |
| Dipole/Dipole: 0.1126 | |
| Total Energy: 17.8985 kcal/mol |
The energy optimised molecule 12 is shown below:
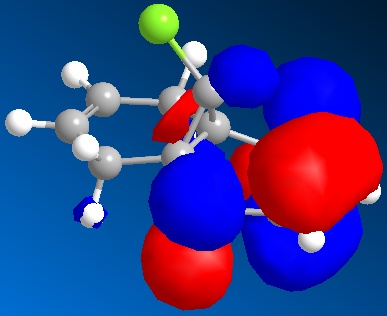
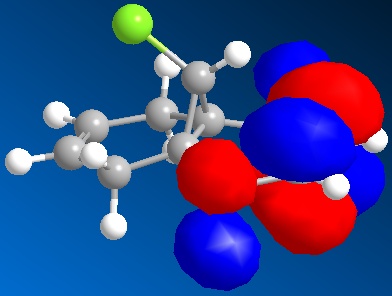
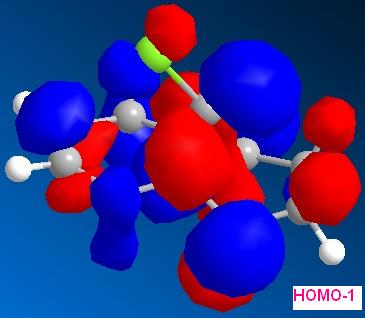
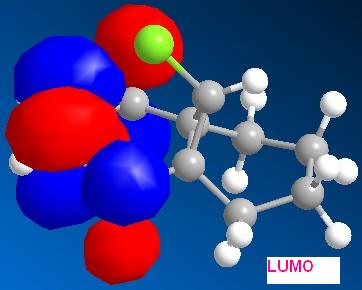
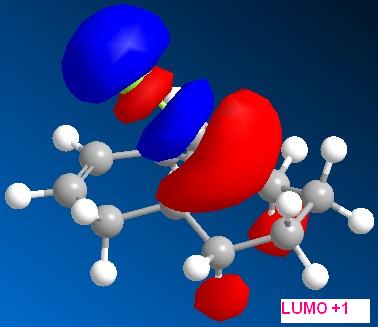
By applying HF/STO-3G self-consistent-field MO method, which used to predict representation of the valence-electron molecular wavefunction, and in particular of the HOMO, presumed to be the most reactive towards electrophilic attack. The molecular orbital representations for LUMO +2, LUMO+1, LUMO, HOMO, HOMO-1 are shown blow, where LUMO+2 represents Cl-C sigma*-orbital, HOMO for endo C=C pi-orbital and HOMO-1for exo C=C pi-orbital.
There is not any distinguishable change between HF/STO-3G self-consistent-field MO method optimised molecule 12 and MM2.
The endo-alkene has larger electron density at the C=C bond at the HOMO orbital compare to exo-alkene, therefore, the endo-alkene is more nucleophilic.
(B) Molecule 13
- the hydrogenated form of molecule 12, analysis the C=C and Cl-C spectroscopic properties.
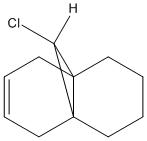 |
Stretch: 0.8331 |
| Bend: 4.9041 | |
| Stretch-Bend: 0.0878 | |
| Torsion: 12.5078 | |
| Non-1,4 VDW: -1.1394 | |
| 1,4 VDW: 7.5182 | |
| Dipole/Dipole: 0.0735 | |
| Total Energy: 24.7851 kcal/mol |
The energy optimised molecule 13 is shown below:
The total energy of molecule 13 is about 7 kcal/mol greater than molecule 12 due to higher torsional strain, which may resulted from the new inducing hydrogen atom and so increasing the diaxial syn-interaction.
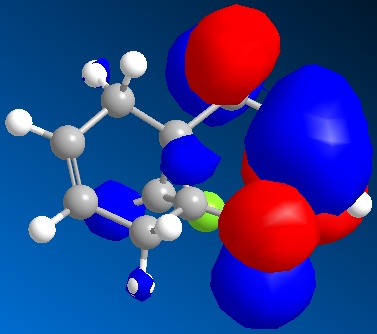
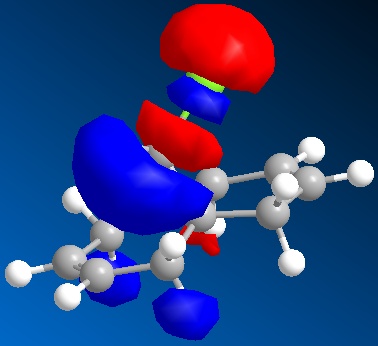
The molecular orbital representations for LUMO +2, LUMO+1, LUMO, HOMO, HOMO-1 are shown blow.
Similiar to molecule 12, There is not any distinguishable change between HF/STO-3G self-consistent-field MO method optimised molecule 13 and MM2.
The molecule obeys a boat conformation with higher energy rather than most optimised chair conformation.
(C) IR - Stretching frequencies
1) Molecule 12
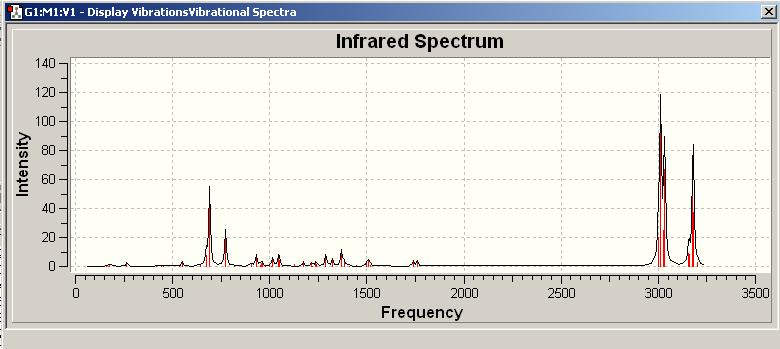
By using GaussView, There were 69 absorption frequency detected in IR of molecule 12.
The distinguishing ones are analysed below:-
| Frequency / cm^-1 | Intensity | Correspondence |
|---|---|---|
| 772.538 | 25.2816 | Cl-C stretching |
| 1740.9 | 4.1547 | Exo C=C double bond stretching |
| 1761.01 | 3.896 | Endo C=C double bond stretching |
The Intensity of Cl-C stretching is much higher than both C=C bond stretching, this is due to the large effect of Cl-C stretching to dipole moment, whereas the C=C stretching only has minor effect on this.
2) Molecule 13
By using GaussView, There were 75 absorption frequency detected in IR of molecule 13.
The distinguishing ones are analysed below:-
| Frequency / cm^-1 | Intensity | Correspondence |
|---|---|---|
| 782.315 | 22.2784 | Cl-C stretching |
| 1757.31 | 5.015 | Endo C=C double bond stretching |
Only Cl-C stretching and endo C=C stretching were detected, no absorption for exo C=C stretching.
3) Analysis of C-Cl stretching frequency
Consider molecule 12, the LUMO+2 (Cl-C sigma*) and HOMO-1 (exo C=C pi-orbital) are pointing towards to each other and hence there is some degree of orbital interaction between them. This interaction leads the electron density donating into the Cl-C sigma* orbital, and therefore, the bond becomes longer and weaker. As a result, the energy required to stretch the Cl-C bond is less and hence the stretching frequency of Cl-C in molecule 12 is smaller. If the hydrogenation occurs at the HOMO (endo C=C pi) orbital, there would be no interaction between this orbital and the LUMO+2 (Cl-C sigma*), and therefore, the stretching frequency will not be effected.
(D) Reference
1. B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
Structure Based Mini Project using DFT-based Molecular Orbital Methods - A Total Synthesis of (±)-Frondosins A and B
(A)Abstract
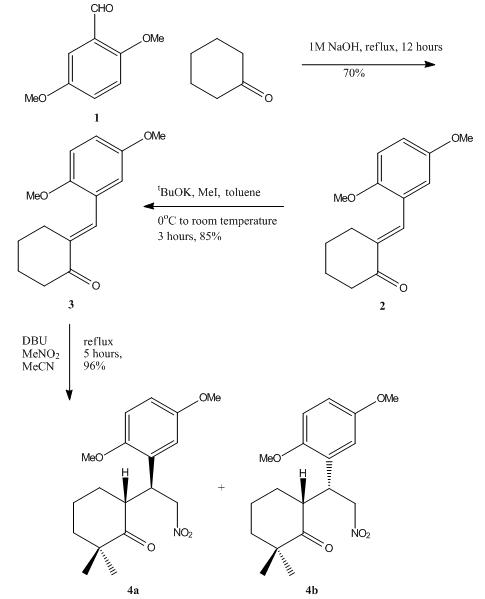
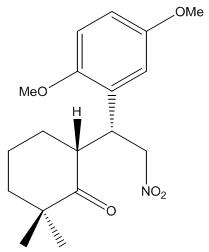
During the total synthesis of bioactive meroterpenoid natural products (±)-frondosins A and B, one of the key synthesis step is the reaction between cyclohexanone and gentistic aldehyde dimethyl ether, and form the following intermediate diastereoisomers shown blow:-
In this report, by using ChemBio 3D and GaussView programmes, we are going to investigate this pair of diastereoisomer by analysis the computational IR, 13C NMR and optical rotation, and also, to compare them with the experimental literature data.
(B) Synthesis of Intermediate Diastereoisomers - Reaction Scheme
Initially, the cyclohexanone was condensed with the dimethyl ether of gentisic aldehyde 1 to furnish arylidene 2, and further exhaustive methylation introduced the gem-dimethyl group present in 3. A Michael addition of the anion derived from nitromethane to 3 led to a diastereomeric mixture (1:1) of 4a and 4b.
(C) Results and Discussions
experimental computational data : experimental value obtained from computational lab session. experimental literature data: values obtained from recent journals.
1) Diastereoisomer 4a
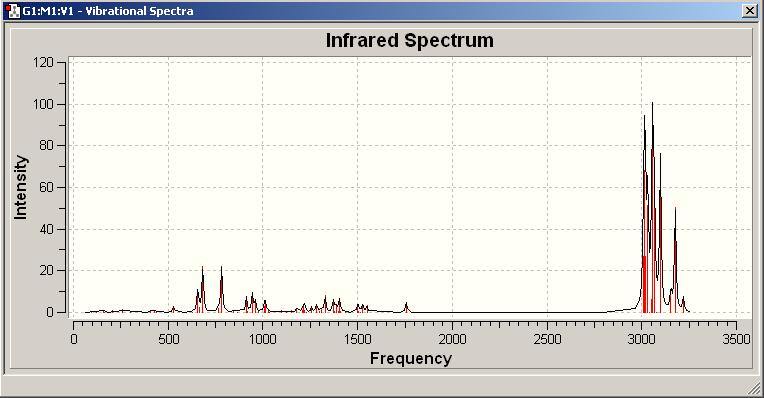
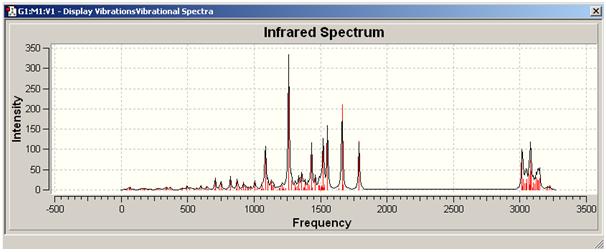
IR spectrum [1]
| Computational Frequency/cm^-1 | Intensity | Assign of FG | Experimental Literature Frequency /cm^-1 |
|---|---|---|---|
| 3022.11, 3011.21 | 58.4313, 63.878 | C-H stretching | 2931 |
| 1778.74 | 134.798 | C=O stretch | 1700 |
| 1654.34, 1289.19 | 217.76, 106.435 | N=O stretch | 1589 |
| 1548.47, 1469.27 | 181.632, 60.8905 | aromatic C=C stretch | 1502 |
| 1436.73 | 69.2652 | C-N stretch | 1463 |
| 817.18 | 26.9807 | aromatic C-H bending | - |
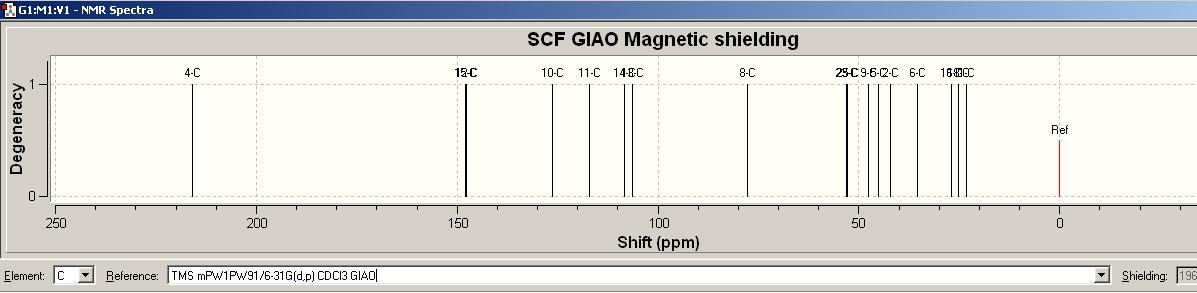
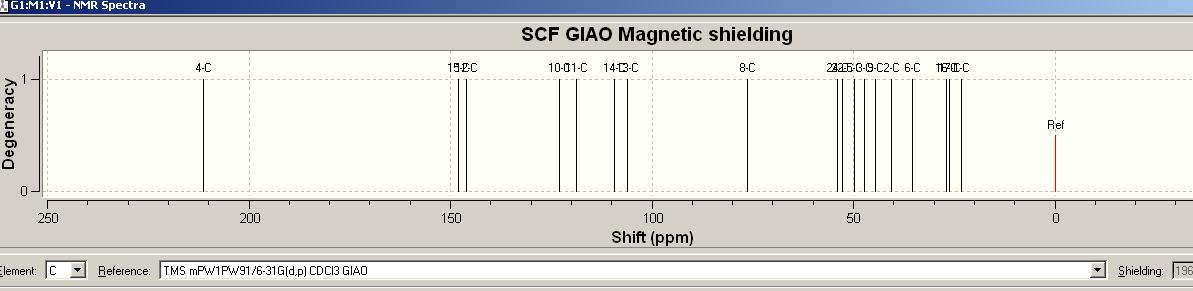
13C NMR spectrum [ http://hdl.handle.net/10042/to-1687]
Experimentally Computational data were obtain by setting ‘TMS mPW1PW91/6-31G(d,p) CDCl3 GIAO ‘ as reference.
| Labelled Carbon No. | Computational Shift/ppm | Experimental Literature Shift /ppm |
|---|---|---|
| 4-C | 216.13 | 216.07 |
| 15-C | 149.02 | 153.51 |
| 12-C | 147.59 | 151.81 |
| 10-C | 126.2 | 127.07 |
| 11-C | 117.06 | 117.27 |
| 14-C | 108.27 | 112.66 |
| 13-C | 107.51 | 111.92 |
| 8-C | 77.67 | 77.61 |
| 23-C | 53.03 | 55.88 |
| 25-C | 52.81 | 55.58 |
| 9-C | 47.69 | 46.12 |
| 3-C | 47.53 | 45.98 |
| 5-C | 45.01 | 42.19 |
| 2-C | 42.14 | 42.19 |
| 6-C | 35.46 | 33.77 |
| 16-C | 27.01 | 25.4 |
| 18-C | 25.17 | 24.56 |
| 1-C | 23.23 | 21.6 |
There are same numbers of carbon assigned in both spectrum. All the chemical shifts from experimentally computational spectrum are match quite well with the experimental literature values, such that the mean shift difference within ±4ppm, which arise from the differences between experimental and theoretical outcomes, and also the inaccuracy in computational modelling.
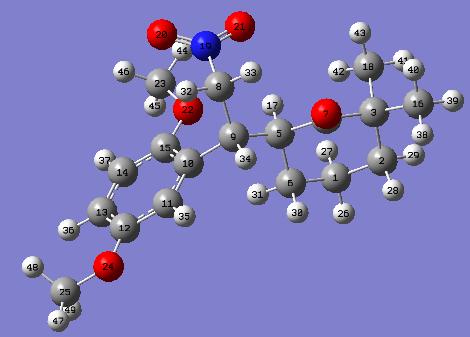
The energy optimised 4a is shown below:
2) Diastereoisomer 4b
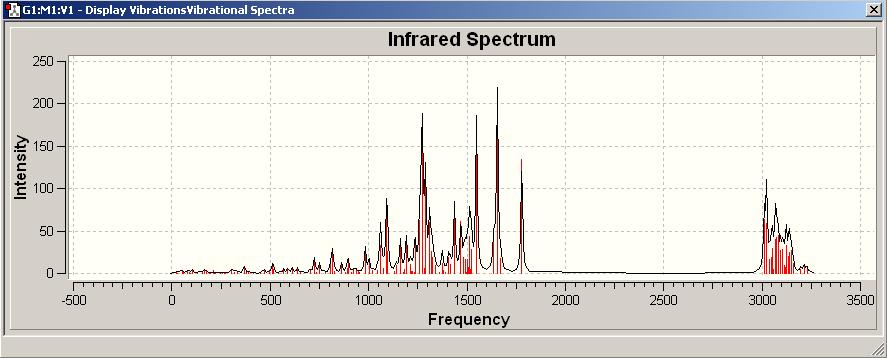
IR spectrum[2]
| Computational Frequency/cm^-1 | Intensity | Assign of FG | Experimental Literature Frequency /cm^-1 |
|---|---|---|---|
| 3016.45 | 101.376 | C-H stretching | 2935 |
| 1789.73 | 119.675 | C=O stretch | 1702 |
| 1661.96, 1260.35 | 209.871, 330.688 | N=O stretch | 1553 |
| 1520.09, 1460.50 | 111.473, 30.4372 | aromatic C=C stretch | 1496 |
| 1432.44 | 108.114 | C-N stretch | 1463 |
| 822.594 | 31.788 | aromatic C-H bending | - |
13C NMR spectrum [error in repository, job title:12723]
Experimentally Computational data were obtain by setting ‘TMS mPW1PW91/6-31G(d,p) CDCl3 GIAO ‘ as reference.
| Labelled Carbon No. | Computational Shift/ppm | Experimental Literature Shift /ppm |
|---|---|---|
| 4-C | 211.22 | 214.85 |
| 15-C | 147.92 | 153.41 |
| 12-C | 145.91 | 151.19 |
| 10-C | 123.14 | 128.39 |
| 11-C | 118.64 | 115 |
| 14-C | 109.13 | 112.01 |
| 13-C | 106.13 | 111.91 |
| 8-C | 76.36 | 75.39 |
| 24-C | 54.09 | 56.01 |
| 22-C | 52.84 | 55.62 |
| 5-C | 49.583 | 47.52 |
| 3-C | 47.33 | 45.79 |
| 9-C | 44.33 | 41.55 |
| 2-C | 40.58 | 36.37 |
| 6-C | 35.32 | 30.23 |
| 16-C | 26.81 | 25.41 |
| 17-C | 26.06 | 24.92 |
| 1-C | 23.31 | 21.48 |
There are same numbers of carbon assigned in both spectrum. All the chemical shifts from experimental computational spectrum are match quite well with the experimental literature values, such that the mean shift difference within ±4ppm. The reasons of deviation are the same as explained for 4a diastereoisomer in previous part.
The energy optimised 4b is shown below:
3) Optical Rotation of Diastereoisomers 4a and 4b
4a: [Alpha]D = 16.49 deg. (http://hdl.handle.net/10042/to-1732)
4b: [Alpha]D = -176.55 deg. (http://hdl.handle.net/10042/to-1731)
For optical rotation, the difference between experimental computational value and experimental literature value are quite large. This difference may brought by a whole set reasons, for example, an inaccurate or even unknown temperature and concentration were used while measuring the experimental literature values. And hence, these 2 sets of data cannot be put together for comparison duo to various measuring condition. However, the positive and negative signs are matched well between them, which indicated the correct enantiomeric configuration of diastereoisomer 4a and 4b.
(C) Reference
1. Goverdhan Mehta, Nachiket S. Likhite, Tetrahedron 49 (2008), P 7113-7116
2. A.D. Patil, A.J. Freyer, L. Killmer, P. Offen, B. Carte, A.J. Jurewicz and R.K. Johnson, Tetrahedron 53 (1997), pp. 5047–5060.
3. H.W. Pinnick, Org. React. 38 (1990), pp. 655–792