Rep:Mod:chocolatefudge9
Contained within these wiki pages is the report by Martin Champion (mjc07) for the Third Year Inorganic Computational Project, Department of Chemistry, Imperial College London (March 2010).
Computational modelling of Inorganic systems
The first part of this project looks how using molecular mechanics approaches (bond lengths and angles) can be used to reasonably approximate real systems and thus how reactivity properties of a molecule(reaction) can be predicted by comparing different geometries of molecules and there isomers.
Modelling and Properties of BH3 and BCl3
BH3
| Calculation Type | opt |
|---|---|
| Method | DFT/B3LYP |
| Basis set | 3-21G |
| Calculation time | 19.0 s |
| Energy (Hartree) | -26.46 |
| RMS Gradient Norm (Hartree) | 0.0002067 |
| B-H Bond length (A) | 1.19 |
| H-B-H Bond angle | 120.0 |
Initial investigations began around optimising the structure of BH3. In Gauss View 5 a trigonal planar Boron unit was drawn and the Hydrogens added automatically. The B-H bond lengths were set at 1.5 A as instructed to[1]. Next a Gausian optimisation calculation was set up using Density functional theory (DFT) with the B3LYP method and
NBO analysis: Charge computed for B: 0.332 and H: -0.111
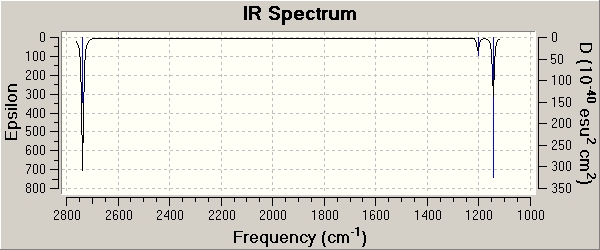
It can be seen from that computed IR spectrum that only three vibrations are shown yet 6 different vibrations have been calculated and displayed in table above. One reason is because there are two sets of Vibrations with E' symmetry thus there two sets of degenerate vibrations. Because of this they will have the same wavelength and thus coincide on the spectrum, so only two peaks are seen instead of four.
The final vibration that is not seen is completely symmetric A1' at 2598 cm-1. It is completely symmetric stretch of all three B-H bonds simultaneously which means there is no change in dipole moment. Stretches or vibrations which exhibit no change in dipole moment are not IR active so won't be displayed in an IR spectrum (IR selection rules). This is also why it is computed to have 0 intensity.
MO
MO calculations of BH3 checkpoint file for mjc07: https://www.ch.imperial.ac.uk/wiki/images/3/3b/Mjc07_bh3_pop.chk
BCl3
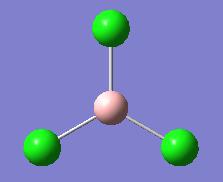
| Calculation Type | opt |
|---|---|
| Method | DFT/B3LYP |
| Basis set | LANL2MB |
| Calculation time | 8.0s |
| Energy (Hartree) | -69.44 |
| RMS Gradient Norm (Hartree) | 0.00005905 |
| B-Cl Bond length (A) | 1.87 |
| Cl-B-Cl Bond angle | 120.0 |
Cis-trans isomerism in tetracarbonyldiphosphinemolybdenum complexes
Modelling structure and properties of Five-Membered Arsenic-Sulfur-Nitrogen Heterocycles
The asymmetry of the ring means in effect there are two chiral isomers of each cycle 1-6 depending whether the R group points up or down (the inversion that is observed for Nitrogen doesn't occur at room temperature for heavier pnictogens elements). There is no immediately obvious reason as to which one might be preferred, or if one is produced exclusively (as is suggested for 5 and 6). The structures of 5 and 6 were given in by the authors in form of x-ray single crystal structures which defines which face the R group is in, but no such information (largely because they did crystallise) thus a guess was made for structures 1-4 as to which face the R group is in.
References
- ↑ Inorganic Comp. Lab Guide., P.Hunt, Dates accessed: 07/03/10-18/03/10., http://www.ch.ic.ac.uk/wiki/index.php/Mod:inorganic