Rep:Mod:chl1718
NH3 molecule
Optimization
| Calculation Method | Basis Set | Final Energy (au) | RMS Gradient (au) | Point Group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -56.55776 | 0.00000485 | C3V |
| Optimized Parameter | Value | Error |
|---|---|---|
| N-H bond distance (Å) | 1.02 | ±0.005 |
| H-N-H bond angle (°) | 106 | ±0.5 |
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000004 | 0.000450 | YES |
| RMS Force | 0.000004 | 0.000300 | YES |
| Maximum Displacement | 0.000072 | 0.001800 | YES |
| RMS Displacement | 0.000035 | 0.001200 | YES |
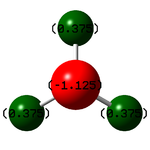
| Atom | Charge |
|---|---|
| N | -1.125 |
| H | +0.375 |
N has a negative charge because it is more electronegative than H.
Optimized molecule |
File:CHL1718 NH3 OPTUF POP.LOG
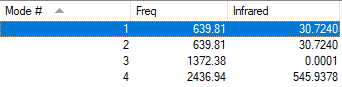
Vibrations
| Bending | Stretching | |||||
| Symmetry | A1 | E | E | A1 | E | E |
| Wavenumber (cm-1) | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Intensity | 145 | 14 | 14 | 1 | 0 | 0 |
The 3N-6 rule correctly predicts 6 modes of vibration from 4 atoms for NH3. There are two degenerate pairs of vibrations; both have E symmetry and are asymmetric. The A1 stretching mode is highly symmetric; the A1 bending vibration is the umbrella mode due to the resemblance to flipping an umbrella.
Overall, there should be 2 bands in a spectrum.
N2 molecule
Optimization
| Calculation Method | Basis Set | Final Energy (au) | RMS Gradient (au) | Point Group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -109.52412 | 0.000004 | D∞h |
| Optimized Parameter | Value | Error |
|---|---|---|
| N-N bond distance (Å) | 1.11 | ±0.005 |
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000006 | 0.000450 | YES |
| RMS Force | 0.000006 | 0.000300 | YES |
| Maximum Displacement | 0.000002 | 0.001800 | YES |
| RMS Displacement | 0.000003 | 0.001200 | YES |
| Atom | Charge |
|---|---|
| N | 0.000 |
Optimized molecule |
Vibrations
| Symmetry | SGG |
| Wavenumber (cm-1) | 2457 |
| Intensity | 0 |
There is only one stretching mode for a diatomic molecule. Since there is no dipole, there is no change in dipole moment so intensity is 0.
N2 Ligand in a Mono-metallic TM Complex
| Reported N-N length (Å) | Calculated N-N length (Å) |
|---|---|
| 1.131 [1] | 1.11 |
The refcode is VEJRUA, an iron complex. Comparing the two types of N2, the N-N triple bond is longer when coordinated to the Fe atom because some electron density ends up in an antibonding orbital of N2 and causes the bond to weaken. Furthermore, the calculation of the N2 optimisation might differ from the true value in the first place, as well as not taking into account the effects of ligand coordination.
H2 molecule
Optimization
| Calculation Method | Basis Set | Final Energy (au) | RMS Gradient (au) | Point Group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -1.17853 | 0.00004 | D∞h |
| Optimized Parameter | Value | Error |
|---|---|---|
| H-H bond distance (Å) | 0.74 | ±0.005 |
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000066 | 0.000450 | YES |
| RMS Force | 0.000066 | 0.000300 | YES |
| Maximum Displacement | 0.000087 | 0.001800 | YES |
| RMS Displacement | 0.000123 | 0.001200 | YES |
| Atom | Charge |
|---|---|
| H | 0.000 |
Optimized molecule |
Vibrations
| Symmetry | SGG |
| Wavenumber (cm-1) | 4464 |
| Intensity | 0 |
See N2
Haber-Bosch energy calculation
| Equation term | E(NH3) | 2*E(NH3) | E(N2) | E(H2) | 3*E(H2) |
| Value (au) | -56.55776 | -113.11552 | -109.52412 | -1.17853 | -3.53559 |
ΔE = 2*E(NH3) - [E(N2) + 3*E(H2)]
ΔE = -113.11552 - (-109.52412 - 3.53559)
ΔE = -0.05581 (au)
ΔE = -146.5 (kJmol-1)
The product NH3 is more thermodynamically stable because the reaction is exothermic.
CO2 molecule
Optimization
| Calculation Method | Basis Set | Final Energy (au) | RMS Gradient (au) | Point Group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -188.58093 | 0.00014 | D∞h |
| Optimized Parameter | Value | Error |
|---|---|---|
| C=O bond distance (Å) | 1.17 | ±0.005 |
| O=C=O bond angle (°) | 180 | ±0.5 |
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000315 | 0.000450 | YES |
| RMS Force | 0.000157 | 0.000300 | YES |
| Maximum Displacement | 0.000192 | 0.001800 | YES |
| RMS Displacement | 0.000118 | 0.001200 | YES |
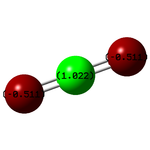
| Atom | Charge |
|---|---|
| C | +1.022 |
| O | -0.511 |
C has a positive charge because oxygen is more electronegative.
Optimized molecule |
File:CHL1718 CO2 OPTUF POP.LOG
Vibrations
| Type | Bending | Stretching | ||
| Symmetry | PI | PI | SG | SG |
| Wavenumber (cm-1) | 640 | 640 | 1372 | 2437 |
| Intensity | 31 | 31 | 0 | 546 |
The rule for linear molecules is 3N-5, so there are 4 vibrational modes for CO2. The two bending modes are degenerate. The stretching mode with 0 intensity is symmetric. Overall, two bands are expected in a spectrum.
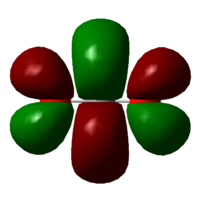
Molecular Orbitals
LUMO (12)
Energy: +0.02997 au
The contributing AO's are 2px orbitals from carbon and oxygen. They are out of phase, so this is an unoccupied π*u antibonding orbital.
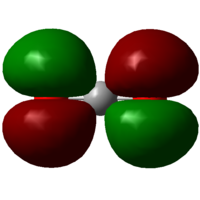
HOMO (11)
Energy: -0.36997 au
The contributing AO's are 2px orbitals from oxygen. Being occupied lone pairs, they do not participate in bonding.
MO 9
Energy: -0.51279 au
The contributing AO's are 2px orbitals from carbon and oxygen. This is an occupied πu bonding orbital, high in energy but below the HOMO.
MO 6
Energy: -0.56232 au
The contributing AO's are 2s orbitals from carbon and oxygen. This is an occupied σg bonding orbital. The lobes are out of phase so there are 2 nodes. This MO is intermediate in energy.
MO 4
Energy: -1.16101 au
The contributing AO's are 2s orbitals from carbon and oxygen. This is an occupied σg bonding orbital and is low in energy.
[NH4]+
Optimization
| Calculation Method | Basis Set | Final Energy (au) | RMS Gradient (au) | Point Group |
|---|---|---|---|---|
| RB3LYP | 6-31G(d,p) | -56.90586 | 0.00000083 | Td |
| Optimized Parameter | Value | Error |
|---|---|---|
| N-H bond distance (Å) | 1.03 | ±0.005 |
| H-N-H bond angle (°) | 109 | ±0.5 |
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000004 | 0.000450 | YES |
| RMS Force | 0.000001 | 0.000300 | YES |
| Maximum Displacement | 0.000003 | 0.001800 | YES |
| RMS Displacement | 0.000002 | 0.001200 | YES |
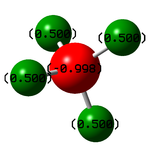
| Atom | Charge |
|---|---|
| N | -0.998 |
| H | +0.500 |
N has a negative charge because it is more electronegative than H.
Optimized molecule |
File:CHL1718 NH4 OPTUF POP.LOG
Vibrations
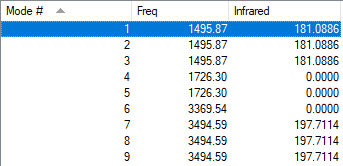
| Bending | Stretching | ||||||||
| Symmetry | T2 | T2 | T2 | E | E | A1 | T2 | T2 | T2 |
| Wavenumber (cm-1) | 1496 | 1496 | 1496 | 1726 | 1726 | 3370 | 3496 | 3496 | 3496 |
| Intensity | 181 | 181 | 181 | 0 | 0 | 0 | 198 | 198 | 198 |
The 3N-6 rule correctly predicts 6 modes of vibration from 4 atoms for NH4+. There are 3 degenerate groups of vibrations. The A1 stretch is symmetric, but the E bends also do not produce a change in dipole so those three have 0 intensity.
Overall, there should be 2 bands in a spectrum.
References
- ↑ Ryuji Imayoshi Kazunari Nakajima Jun Takaya Nobuharu Iwasawa Yoshiaki Nishibayashi (2017) Synthesis and Reactivity of Iron– and Cobalt–Dinitrogen Complexes Bearing PSiP‐Type Pincer Ligands toward Nitrogen Fixation, European Journal of Inorganic Chemistry, 2017, 32, 3769-3778. Available from https://onlinelibrary.wiley.com/doi/full/10.1002/ejic.201700569
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES - well done for explaining the origins of the charges. Your MO explanations are very good overall. I would consider MO6 to be anti-bonding between the C and O atoms, because (as you pointed out) there are nodal planes intersecting the bonds between the atoms.
See this wiki page for more details on the CO2 MOs.
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
You did an extra calculation with some good analysis on NH4+ well done!
Do some deeper analysis on your results so far