Rep:Mod:chem2010a
MODULE 1
INTRODUCTION
Using ChemBio3D and Gaussview it is possible to model different aspects of molecules reactivity and structure without the need to do the reaction in the laboratory. Using this technique the major product of a reaction can be predicted and modifications to the structure can be made. Theoretically any conceivable molecule could be modelled in this way. There are however some limitations to using molecular mechanics to model molecules - as will be undertaken for the first half of this exercise. Molecular mechanics needs to use data from well known and well documented molecules, so the reactions that are modelled using this technique cannot be too different from modern documented molecules. For this reason, if the molecule has unusual bonding then the results produced from the molecular modelling will be full of inaccuracies. In addition to this some ions cannot be accurately modelled.
Modelling using Molecular Mechanics
The Hydrogenation of a Cyclopentadiene Dimer
When Cyclopentadiene dimerises it produces one of two different dimers - either the exothermic or the endothermic dimer. Dimer 1 is the exo dimer and dimer 2 is the endo dimer, which is proven by the different total energies of dimer one and dimer two, dimer 2 has a higher total energy and is therefore less stable and thus the kinetic rather than the thermodynamic product. However, as the endo product is the product that is formed then the formation of the cyclopentadiene dimer is kinetically controlled. The endo rule for the DIels Alder reaction can be explained by the use of the orbitals; the frontier orbitals come together in a sigma bonding manner at the 'front' of the orbital. When the secondary orbitals are also taken into consideration they too overlap in what would be a bonding manner however this overlap does not lead to the formation of a bond but instead the sum of all the favourable interactions leads to the endo product being formed over the exo product[1].ead the molecular modelling cannot take into account frontier orbitals. The program seems to predict that the exo product would be formed instead because it does not use a quantum mechanical approach and inst The mechanism of the reaction is as follows:

Dimer 1 looks like the following:
| ACTION | DIMER 1: ENERGY kcal/mol |
|---|---|
| STRETCH | 1.3003 |
| BEND | 20.5955 |
| STRETCH-BEND | -0.8463 |
| TORSION | 7.6334 |
| 1,4 NON VdW INTERACTIONS | -1.3992 |
| 1,4 VdW INTERACTIONS | 4.2244 |
| DIPOLE-DIPOLE | 0.3771 |
| TOTAL ENERGY | 31.8852 |
Dimer 2 looks like the following:
| ACTION | DIMER 2: ENERGY kcal/mol |
|---|---|
| STRETCH | 1.2376 |
| BEND | 20.8420 |
| STRETCH-BEND | -0.8301 |
| TORSION | 9.5095 |
| 1,4 NON VdW INTERACTIONS | -1.5158 |
| 1,4 VdW INTERACTIONS | 4.3183 |
| DIPOLE-DIPOLE | 0.4459 |
| TOTAL ENERGY | 34.0074 |
Dimers 3 and 4 look are as follows:
| ACTION | DIMER 3: ENERGY kcal/mol | DIMER 4: ENERGY kcal/mol |
|---|---|---|
| STRETCH | 1.2659 | 1.0952 |
| BEND | 19.8063 | 14.5166 |
| STRETCH-BEND | -0.8276 | -0.5473 |
| TORSION | 9.5095 | 12.5056 |
| 1,4 NON VdW INTERACTIONS | -1.5158 | -1.0617 |
| 1,4 VdW INTERACTIONS | 4.3183 | 4.5088 |
| DIPOLE-DIPOLE | 0.1621 | 0.1406 |
| TOTAL ENERGY | 35.6953 | 31.1578 |
When the different alkene bonds in dimer 2 are hydrogenated it produces two different molecules. The energy of dimer 3 is higher than that of dimer 4, thus dimer 3 is the kinetic result of the reaction and dimer 4 is the thermodynamically more likely result.
STRETCH - For dimer 4 the stretch in the molecule is less than for dimer 3, suggesting a more restricted conformation, this is due to the restricted ring inversion in dimer 4. In dimer 3 the pentane ring can invert while the hydrogens stay axial. Whereas in dimer 4 with the further restriction of the alkene in the 5-membered ring there can be less stretching of the bonds.
BEND - Dimer 3 has more of a bend to the molecule as the conformation is less restricted.
TORSION - There is a higher torsion in the bonds for dimer 4 than there is for dimer 3 which suggests that the conformation of dimer 4 is more 'twisted' than that for dimer 3.
VAN DER WAALS INTERACTIONS - The Van der Waals interactions for the dimer 4 are higher than for dimer 3 as the hydrogen molecules interact with the bridge over the 6 membered ring and also the orbitals on the alkene in the 5 membered ring.
In total all of the interactions and bond energies for dimer 3 contribute in raising the total energy of the molecule. This is because the double bond in the norborene causes strain and so when it is hydrolysed to form molecule 4 the tension in the molecule is released making it a lower energy. However, the alkene in the cyclopentene ring attached to the norborene does not cause such strain in the molecule, thus, when it is hydrogenated the lowering of energy is not as great.
Stereochemistry of Nucleophilic Additions to a pyridinium ring (NAD+ analogue)
When the optically active prolinol reacts with MeMgI to alkylate the 4 position of the pyridine ring it produces the following mechanism:

When using ChemBio3D to to the MM2 minimalisation of energy, the MeMgI group is not taken into account because the program uses the molecular modelling technique and is unable to accurately represent metal ions so ChemBio3D cannot simulate the magnesium ion in a realistic fashion.
The N-methyl derivative of Pyridoxazepinone undergoes highly stereoselective and regiselective addition using the Grignard reagent (MeMgI), which gives the major product as is shown in the above mechanism. However, a minor product can be formed where the methyl group is added ortho to the Nitrogen in the N-methyl 6 membered ring.

The high regio and stereoselectivity comes from the coordination between the Grignard and the oxygen. As has been seen in the article by Schultz and Flood [2]
| Dihedral angle | 11.2 | 10.2 | 23.7 |
|---|---|---|---|
| TOTAL ENERGY | 43.1267 | 43.0562 | 44.7149 |
When the geometry of the molecule was altered in ChemBio3D for most of the manipulations the lowest energy conformation constantly returned to have a dihedral angle of approximately 11 degrees for the carboxyl bond when the angle was measured between the carboxyl to the double bond in the six membered ring, rather than into the five membered ring. One other conformation had a reasonable minimum energy when the dihedral angle is 23.7 degrees. However, the total energy for the second conformation is approximately 1.6kcal/mol higher than the first conformation. Suggesting that the major product would be the first conformation, as the angle is always positive this suggests that when the Grignard agent reacts with the carbonyl group it attacks from above the plane of the ring. In both conformations the 5 membered rings are both bent in a near boat conformation, the real difference comes with the analysis of the 7 membered ring where the carbonyl group can either be coplanar with the Nitrogen in the 5 membered ring, or the carbonyl can be below the plane of the ring which is a slightly higher energy conformer.
For molecule 7 there appears to be only one product formed with no other isomers despite attempts to drastically alter the structure in ChemBio3D:

| Dihedral angle | -20.15 | 21.13 |
|---|---|---|
| TOTAL ENERGY | 63.4436 | 63.4436 |
All attempts on this molecule produced identical energies, the only variation was whether the NPhH group was added above the plane of the six membered ring or below the plane of the six membered ring. In the literature it is suggested that there is only one product formed as an anchoring step may be introduced to hold the conformaiton of the molecule and limit the isomers. However, when the simulation is run in ChemBio3D no such anchor is used so both atropisomers are produced, which explains why they both have the same total energy. [3]:
 |
 |
|---|---|
| Above the plane | Below the plane
|
Stereochemistry and Reactivity of an intermediate in the synthesis of Taxol
When Taxol - an important cancer medication - is being synthesised, there is an intermediate formed which either has the carbonyl group above the ring or below the ring. When the intermediates are left to stand then the least stable atropisomer alters and adopts the more stable form, the energies for the modifications of the molecules show which one is the most stable isomer:
| Molecule 9 | Molecule 10 | |||||
|---|---|---|---|---|---|---|
|
|
| |||||
| TOTAL ENERGY | 49.4461 kcal/mol | 59.2666 kcal/mol |
When the Carboxyl group is down the energy of the molecule is lower - even when the energy conformation of the molecule is at its minimum. When the Carboxyl group is above the plane of the ring then the energy of the conformation is significantly higher. It is know that when this molecule[4] undergoes the Oxy-Cope mechanism it produces antropisomers by rotating the carbonyl group. Molecule 9 is a lower energy than that of molecule 10 because the 6 membered ring is in the chair conformation in molecule 9 which is the lowest possible energy state for it to be in. However, in molecule 10 the conformation of the 6 membered ring is a twist-boat conformation which is unstable and hence has a high energy, which contributes to the high overall energy of the molecule.
The molecule is slow to react because the alkenes are hyperstabilised and thus inert to hydrogenation if they are close to the bridgehead in the Taxol. The hyperstable olefins have a lower strain energy than that of the original bond and so their hydrogenation energies are lower, which makes them less inclined to undergo hydrogenation, thus slowing the rate of the reaction - this can be found for most medium sized ring systems.[5] [6][7]
Modelling Using Semi-Empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
In this section of the report rather than the ChemBio3D using MM2 to minimise the energies of the molecules - where it uses a database of known bond lengths, minimised torsion energies, bond stretches and Van der Waals interactions for the specific molecular environments and then uses all this information to rearrange the shape of the molecule. In this section in addition to MM2 energy minimisation, MOPAC is also used to take into account the electronic configuration of the molecules and show the molecular orbital interactions.
 |
 |
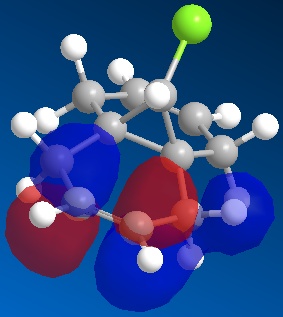 |
 |
 |
|---|---|---|---|---|
| HOMO | HOMO -1 | LUMO | LUMO +1 | LUMO +2 |
The endo alkene in the dichlorocarbene molecule is believed to be more reactive because of electrostatic elements which mean that the exo alkene is much more reactive.[8] This analysis of the reactivity of the alkenes present can be understood by looking at the above representations of the molecular orbitals. It can be seen that the antibonding sigma orbital of the chlorine in the HOMO-1 overlaps with the pi bonding orbital of the LUMO +2 orbital. This overlaps stabilises the bond, lowering it in energy and thus making it less likely to hydrogenate. The exo alkene is lower in energy by 0.08eV than the endo alkene and is shown in the literature by Rzepa, Halton and Boese, this makes the endo alkene more susceptible to electrophiles.
Vibrational Frequencies of the products
| Molecule | C-Cl | anti alkene | syn alkene |
|---|---|---|---|
| dichlorocarbene | 772.597 | 1740.85 | 1760.99 |
| syn alkene (hydrogenated) | 776.823 | - | 1761.66 |
| Optional (with OH group) | 767.333 | 1756.49 | 1761.21
|
In this section of the investigation into molecule 12 the different stretching frequencies of the C=C bonds for the syn and the anti alkenes in the molecule have been taken, as have the stretching frequencies for the C-Cl bonds. These different frequencies have been looked at for the syn hydrolysed alkene variant of the molecule and an optional version of molecule 12 where an electron withdrawing -OH bond has been attached to the anti alkene. When the different stretching frequencies are analysed it can be seen that there is not much variation at all between the C-Cl stretches which all match the theoretical value where the stretch is predicted to be in the 600-800 cm-1 range[9].
The C-Cl stretching frequencies alter slightly as the alkenes are hydrolysed; so, as an -OH group is added to the anti-alkene. This reduction in strength of the C-Cl stretch may occur because the -OH group is electron withdrawing and so will reduce the pi electron density in the alkene bond, thus reducing the strength of the C-Cl bond, which takes its strength from the overlap from the anti-bonding Cl sigma orbital with the pi orbital of the anti-alkene. The strength of the anti-alkene stretch increases (E=hf) because of increased resonance donation from the lone pairs on the oxygen of the -OH (which is a larger contribution than its electronegative electron withdrawing characteristics). This then increases the electron density in the pi cloud so the alkene bond becomes stronger.
All of the C=C bond stretching frequencies are higher in energy than is expected from the literature values of 1620-1680cm-1[10] This increase in the values for the stretching frequencies is due to inaccuracies arising from the model used to simulate the stretches. The model that is used does not use a detailed enough basis set to show all the different forces acting on the molecule. Should a more detailed basis set have been used then the time required to run the simulation would have been much longer and this amount of detail is not required for this specific project so minimising computation time was taken in preference to an increased accuracy in results.
MINI PROJECT
This project is based on the journal by Liu, Sun, Miyazaki, Liu, Wang and Xi - Synthesis of multiply substituted alkylidene silacyclohexadiene derivatives via Palladium-catalysed insertion of alkynes into alkylidene silacyclobutenes.[11]. In this journal the researchers used a palladium catalysts to react a silacyclobutene ring with alkynes. The cycloaddition gives many different regioisomers. The 4 membered ring of the silacyclobutene ring has high ring tension which makes it particularly susceptible to cycloaddition by the alkyne. The 4 membered ring has high thermal and kinetic stability because of the conjugation of the exo alkylidene:

Using Path A involves cycloaddition using the aC-Si bond to form the product which is known in the literature as 4ga. When Path B is used - where the cycloaddition occurs across the bC-Si bond then the product 4gc is formed. There is the potential for two other regioisomers to be formed, however these are unlikely to be formed as

these regioisomers are a higher energy than the regioisomers which are formed. The energies of the different regioisomers are:
| Path A regioisomer | Path A potential regioisomer | Path B regioisomer | Path B potential regioisomer |
|---|---|---|---|
| 47.826 kcal/mol | 54.2741 kcal/mol | 52.9375 kcal/mol | 63.6812 kcal/mol
|
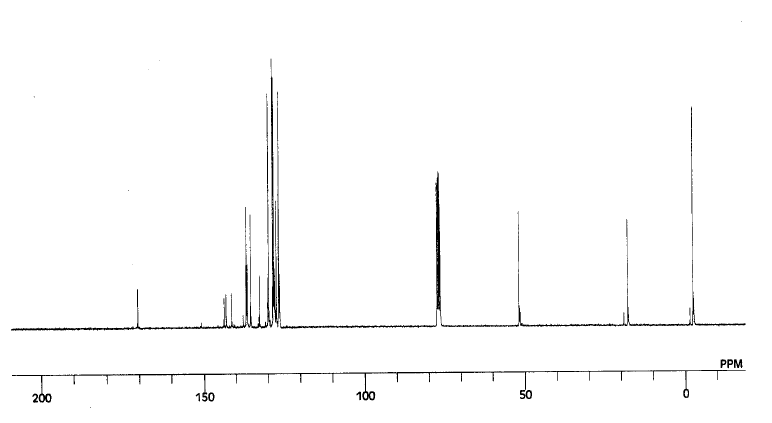
The NMR's for each of the different regioisomers are taken and produce the following spectra:
For molecule 4ag:


For molecule 4gc:


The peaks correspond to the following values for the C13 NMR ppm:

| Atoms 4ag | Atoms 4gc | NMR 4ag | NMR 4gc |
|---|---|---|---|
| 1 | 1 | 145.38 | 128.50 |
| 2 | 2 | 137.46 | 140.06 |
| - | 3 | - | 141.00 |
| 4 | 4 | 152.17 | 142.99 |
| 5 | - | 137.46 | - |
| 6 | 6 | 130.45 | 132.62 |
| 7 | 7 | 140.35 | 142.75 |
| 8 | 8 | 130.73 | 133.96 |
| 9 | 26 | 127.72 | 127.31 |
| 10 | 25 | 124.10 | 124.32 |
| 11 | 24 | 125.46 | 124.70 |
| 12 | 23 | 123.69 | 124.97 |
| 13 | 22 | 128.90 | 126.50 |
| 14 | 10 | 162.78 | 164.91 |
| 17 | 16 | 54.66 | 50.81 |
| 18 | 9 | 20.43 | 20.87 |
| 19 | 11 | 140.24 | 141.22 |
| 20 | 17 | 122.22 | 122.97 |
| 24 | 21 | 124.10 | 123.96 |
| 23 | 20 | 124.47 | 125.12 |
| 21 | 18 | 125.57 | 124.15 |
| 22 | 19 | 123.16 | 123.46 |
| 25 | 12 | -2.73 | -3.25 |
| 26 | 13 | 0.87 | -0.64 |
The above graph can be used to differentiate between the different regioisomers. The graph makes differentiating between the 4ag regioisomer and the 4gc isomer easy because when the literature NMR is viewed it is clearly visible that most peaks have two peaks. One on the right hand side which has an area under the graph seven times greater than the peak to the left - these peaks are most visible in the lower ppm region of the spectrum because of the lack of overlap of the different peaks in this region. In the literature the mixture of regioisomers which are inseparable contains a ratio of 7:1 of the products. In the separated NMRs the signals which are shifted more to the left are from the 4gc regioisomer which correspond to the smaller peaks. Most of the peaks are shifted slightly to the left because the through space interactions deshield the nuclei and so the shift is more upfield. Whereas, in the 120-150ppm region of the spectrum - the region of the spectrum reserved for the aromatic peaks[12]. With two phenyl groups attached to the system there are many different overlaps in this region of the spectra and that makes the different peaks difficult to analyse. However, it can be seen [13] that if the two simulated molecules NMR's are superimposed on top of each other then it closely resembles the literature NMR :
Because of the high levels of overlap on the NMR spectra which all overlap causing multiplets it is impossible to compare all the literature value peaks and the calculated peaks to analyse any differences and see whether the simulation is particularly accurate. However this has been attempted on the individual peaks that are not in the aromatic region:
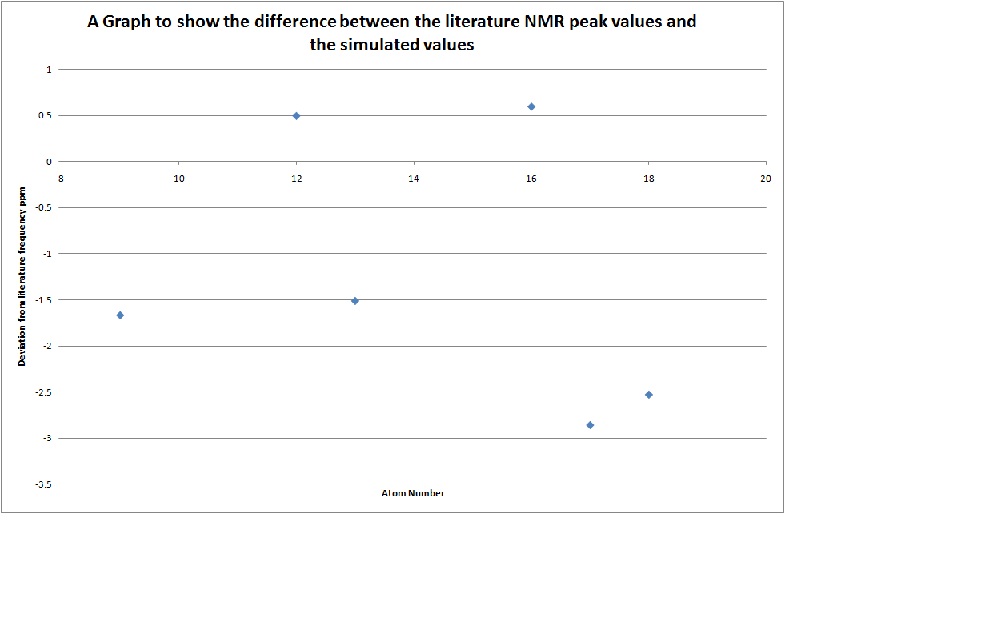
The graph shows that the simulated peaks are never exactly the same as the literature values and instead they fluctuate about the values. This is because of the basis set used to calculate the spectra needs to be more accurate; if this was the case then this would minimise the fluctuations in values until the differences approached zero. This would require a more detailed basis set and a longer running time on the computer. There is another anomaly in the calculated peaks in that in the NMR for the 4ag isomer there is a peak of double the intensity of all the other peaks, this has occurred because - purely by circumstance - the 5 carbon which is attached to an ester group and so is deshielded and is placed at 137.46ppm, in addition to this the carbon 2 atom which is attached to a phenyl group and then to a Si atom is shifted to the same ppm of 137.46ppm.
The only way to see which regioisomer is created is by looking at the molecules 1,2,3,4,5 and 6 rather than the other atoms in the molecule as these are all mainly in the aromatic region of the spectrum and so resonate with each other. Most of the differences between the isomers show only shifts of 2ppm, enough to distinguish which is the major or minor product but only in the lower region of the spectrum. However, when atoms 1 and 4 are considered then the differences in the calculated peaks are clearly visible.
| Atoms 4ag | Atoms 4gc | NMR 4ag ppm | NMR 4gc ppm | Differences |
|---|---|---|---|---|
| 1 | 1 | 145.38 | 128.50 | 16.88 |
| 4 | 4 | 152.17 | 142.90 | 9.27 |
The differences in values are because of the different locations of the atoms. Although at first glance the environments appear to be similar, there are different resonaces which can occur. However, because of the close proximity of these shifts to the aromatic shifts it is impossible to distinguish them by using the literature NMR:

Although it is impossible to confirm the different isomers from the literature the increased resonance in molecule one would appear to make it the major product as it is lower in energy (values are displayed in the table above) but the peaks are impossible to isolate from the C13 NMR.
The literature states that the reaction takes 24 hours to complete, the reaction is slow because of the use of unsymmetrical alkynes and the steric hindrance of the ester group. If the ester was COOPh rather than COOMe the reaction would take even longer. Molecule 4ag is the major product of the cycloaddition reaction because of the allylic character of the Si-aC bond, which makes the bond itself longer and thus weaker and more susceptible to attack by the alkyne than the much less reactive vinyl Si-bC bond, [15] so much so that the ratio for the products is 7:1.
Conclusion
To conclude, in this module the methods of molecular modelling and semi empirical molecular orbital theory have been used to analyse the different mechanisms to see why specific isomers are created in preference to others. For example in the modelling of the cyclopentadiene dimer the exo product is predicted using the MM2 method, however the endo product is the rational product for the reaction. The MOPAC simulation is needed to simulate the different molecular orbitals and by using these to visualise the different orbitals the products can be rationalised. The mini project considers the cycloaddition of an alkyne to silacyclobutenes. In the project it was considered that by analysing the C13 NMR spectra the different regioisomers could be identified and separated out from the spectra given in the literature of the mixture of isomers. However, when the calculations were undertaken the two chemical shifts which would make it possible to identify the different molecules in the spectra merged into a highly populated region of the spectra making the project inconclusive. The general improvements that can be made to the simulations could be that the basis sets could be made more detailed which would improve the results produced by the different simulations, making the results even closer to literature values.
REFERENCES
- ↑ Clayden, Organic Chemistry, 2008, page 916-917
- ↑ Schultz, Flood and Springer, J. Org. Chem., 1986, Regio- and Stereoselective Control in the Addition of Grignard Reagents to the Pyridine Ring System[[1]
- ↑ Leleu, Papamicael, Marsais, Dupas and Levacher, Tetrahedron Asymmetry, 2004, Preparation of axially chiral quinolinium salts related to NAD+ models: new investigations of these biomimetic models as chiral amide-transferring agents [2]
- ↑ Elmore and Paquette, Tetrahedron Letters, 1991, The first Thermally induced retro-oxy-cope rearrangement[[3]
- ↑ McEwen and Paul von Rague Schleyer,J. Am. Chem. Soc., 1985, [[4]]
- ↑ Py, Harwig, Banerjee, Brown and Fallis, Tetrahedron Letters, 1998, Taxamycin studies: Synthesis of taxoid-calicheamicin hybrids [5]
- ↑ Shea[6]
- ↑ Halton, Boese and Rzepa, J. Chem. Soc., 1992, A Molecular Orbital and Crystallographic Study of the Structure and 7t-Facial Regioselectivity of 9-Chloro -1,4,5,8- tetrahydro-4a ,8a - methanonaphthalene[7]
- ↑ IR bond stretches[8]
- ↑ IR bond stretches[9]
- ↑ Liu, Sun, Miyazaki, Liu, Wang and Xi - Synthesis of multiply substituted alkylidene silacyclohexadiene derivatives via Palladium-catalysed insertion of alkynes into alkylidene silacyclobutenes.[[10]]
- ↑ Chemical shifts in carbon NMR data[[11]]
- ↑ Liu, Sun, Miyazaki, Liu, Wang and Xi - Synthesis of multiply substituted alkylidene silacyclohexadiene derivatives via Palladium-catalysed insertion of alkynes into alkylidene silacyclobutenes.[[12]]
- ↑ Liu, Sun, Miyazaki, Liu, Wang and Xi - Synthesis of multiply substituted alkylidene silacyclohexadiene derivatives via Palladium-catalysed insertion of alkynes into alkylidene silacyclobutenes.[[13]]
- ↑ Clayden, Organic Chemistry, 2008, page 41
