Rep:Mod:chaerina
Module 2 - Molecular Modelling to Determine the Structure and Stereoelectronic Properties of Simple Molecules
Exercise 1 - Boronic Compounds
A Molecule of BH3 and BCl3
Introduction
Molecular Modelling is a method that employs Quantum Mechanical approximations to converge into an optimised structure that is as close to reality as possible.
Programs like Gaussian use so-called "basis sets" in order to piece together the relevant units that resemble the constituent nuclei and electron distributions and converge to optimisation.
The basis sets themselves vary in terms of sophistication, which has some bearing on the quality of the resulting optimisation. Be it the case that a more sophisticated basis set returns a more accurate representation of a molecule's structure, the time taken to reach the level of optimisation required would necessitate a longer period of time. As such, a compromise between the quality of optimisation and the amount of time to reach optimisation. With smaller molecules, a larger basis set may be used, however, with larger molecules, larger basis sets would result in extended computing times.
The optimisation of BH3
BH3 was optimised by way of DFT method B3LYP basis set 3-21G, returning the following values for the bond lengths and bond angles:-
| Bond Length (Å) | Bond Angle (o) |
| 1.19 | 120.0 |
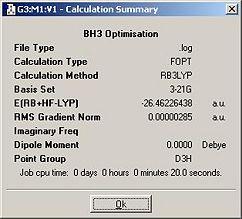
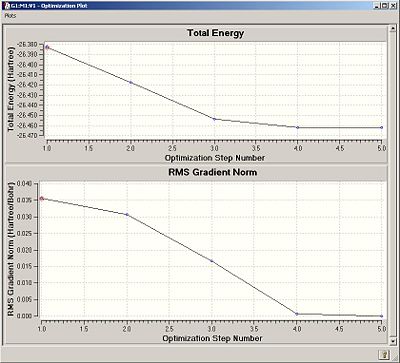
The summary of the Gaussian job was given by the following window and charts:-
|
|
| Question | Answer |
| What is the file type? | .log |
| What is the calculation type? | FOPT |
| What is the calculation method? | RB3LYP |
| What is the basis set? | 3-21G |
| What is the final energy in a.u. (atomic units)? | -26.46 |
| What is the gradient? | 0.00000285 |
| What is the dipole moment? (debye) | 0.0 |
| What is the point group? | D3H |
The graphs shown here represent the energy and the gradient of the energy throughout the process of optimisation. Convergence of the molecular structure is indicated here by the fact that the energy is at the lowest and the gradient is at its lowest at the end of optimisation, as seen in the graphs. It should be noted that the starting B-H bond length was set to 1.5Å and was optimised to the recorded value of 1.19435Å. The placement of bonds by Gaussview is dependent on a set distance between atoms. Further than this distance, gaussview does not indicate the existence of a bond.
| Stage in Optimisation | ||||
 |
 |
 |
 |

|
| Bond Length (Å) | ||||
| 1.50 | 1.41 | 1.28 | 1.19 | 1.19 |
As can be seen from the optimisation profile, gaussview only draws in the bonds at stage 4; the existence of a bond is not acknowledged prior to that stage. It is important to respect the fact that formal bonding is seen as a direct linkage between atoms, which is purely a stylistic representation of a REAL chemical bond. In reality, and as expressed in molecular orbital terms, a chemical bond is represented by the electron cloud between the two atoms and its shape in comparison to the atomic case.
Molecular Orbital (MO) and Natural Bond Orbital (NBO) Analysis of BH3
Gaussian can also predict the electronic interactions of the atoms within the molecule, building up a molecular orbital picture of the entire molecule.
The MOs are visualised as lobes with varying phase, the MOs obtained for BH3 are as follows:-
| BH3 Molecular Orbitals | |
| MO | MO Type |
 |
A1` |
 |
A1` |
 |
A2`` |
 |
HOMO, E` |
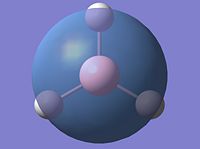 |
LUMO, A1` |
The NBO approach analyses the charge distribution of the molecule and is in some ways a surmising method of the molecular orbital theory method.
The scale is to the effect of the following table:-
| NBO Analysis of BH3 |
| Colour Representation |

|
| Numerical Representation |

|
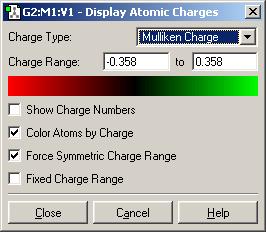
|
The table shows a scale of charge that ranges from very negatively charged (red) to very positively charged ( green). As can be seen in the diagram of BH3, the Boron is highly positively charged, whereas the Hydrogens are moderately negatively charged. This is consistent with reality as boron is very lewis acidic, being electron deficient and having a high charge/mass ratio.
Although this gives a clear picture of the relative charges of the individual atoms, the diagram is highly stylised once again, and says nothing on the electronic level. A table of orbital contributions and ratios is also given by gaussview in the NBO analysis:-
| Bonding Orbital Contributional Analysis |
(Occupancy) Bond orbital/ Coefficients/ Hybrids
---------------------------------------------------------------------------------
1. (1.99851) BD ( 1) B 1 - H 2
( 44.49%) 0.6670* B 1 s( 33.33%)p 2.00( 66.67%)
0.0000 0.5774 0.0000 0.0000 0.0000
0.8165 0.0000 0.0000 0.0000
( 55.51%) 0.7451* H 2 s(100.00%)
1.0000 0.0000
2. (1.99851) BD ( 1) B 1 - H 3
( 44.49%) 0.6670* B 1 s( 33.33%)p 2.00( 66.67%)
0.0000 0.5774 0.0000 0.7071 0.0000
-0.4082 0.0000 0.0000 0.0000
( 55.51%) 0.7451* H 3 s(100.00%)
1.0000 0.0000
3. (1.99851) BD ( 1) B 1 - H 4
( 44.49%) 0.6670* B 1 s( 33.33%)p 2.00( 66.67%)
0.0000 0.5774 0.0000 -0.7071 0.0000
-0.4082 0.0000 0.0000 0.0000
( 55.51%) 0.7451* H 4 s(100.00%)
1.0000 0.0000
4. (1.99904) CR ( 1) B 1 s(100.00%)
1.0000 0.0000 0.0000 0.0000 0.0000
0.0000 0.0000 0.0000 0.0000
|
This table shows the contributions from either atom towards each "natural bonding" orbital and their respective hybridisations. It can be seen that the first three are identical in terms of ratio and hybridisation, and represent each of the 3 B-H bonds. The 4th NBO has no contribution from hydrogen and is thus the boron 1s orbital. In the first 3, the orbitals of boron are one third s-character and two thirds p-character, whereas with hydrogen there is no hybridisation.
This is consistent with the idea that the boron is sp2 hybridised, on account of its bonding order and angle, thus having the reported ratios of orbital character in its hybridisation.
Structural analysis of BCl3
In this exercise, a structurally-similar Boron Chloride (BCl3) (see below) was taken and specified a point group of D3h before having its structure optimised.
In comparison to BH3, which has a bond length of 1.19Å, BCl3 has a bond length of 1.87Å, which is notably longer. This may be rationalised by the fact that the B_Cl bonds are more polar due to the larger electronegativity difference between boron and chlorine. The electron withdrawing nature of chlorine siphons electron density from the bond, thereby making it weaker and therefore longer. Both species are totally symmetric, however, and share a bond angle of 120o.
Both species need to optimised using the same method and basis set. This is so that their optimised structures may be reliably compared under the same environment and constituent units.
A frequency analysis was also utilised:-
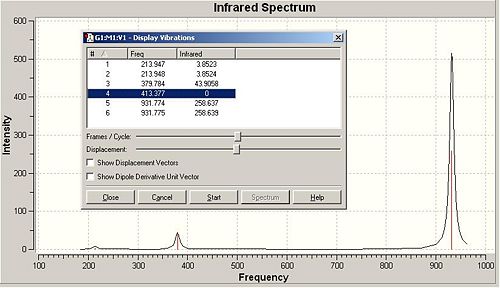
|
In order for a minimum to be reached, the frequencies of the stretches and bend of the molecule must all be positive. From this frequency analysis, one can know that the proposed structure is a minimum.
Exercise 2 - Cis/Trans Isomerisation in a [Mo(CO)4(PCl3)2]
There are 2 possible stereoisomers for Dicarboxytetraphosphoniumchloride Molybdenum [Mo(CO)4(PCl3)2], as detailed below:-
| Isomers of [Mo(CO)4(PCl3)2] | |
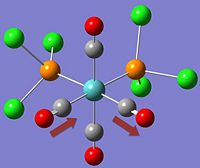
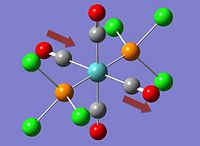
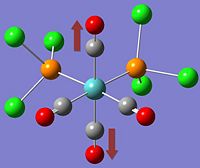
| Cis- | Trans- |
 |
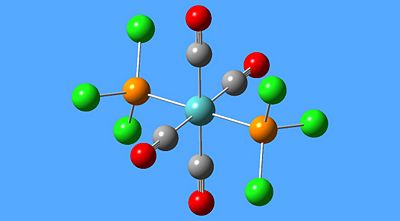
|
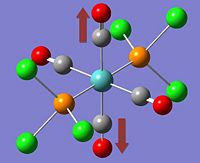
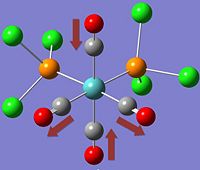
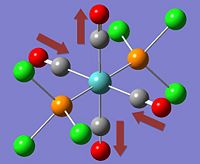
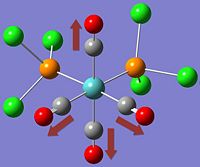
The two stereoisomers can be distinguished by means of Vibrational Frequency Analysis, or in this case, its simulation and paying special attention to the carbonyl stretches within the complex.
The frequency data are presented below:-
*NB:- The arrows here correspond to the carbonyl OXYGEN's movement in relation to the carbon.
The following table was also returned from the frequency analysis:-
| CIS-[Mo(CO)4(PCl3)2] | TRANS-[Mo(CO)4(PCl3)2] |
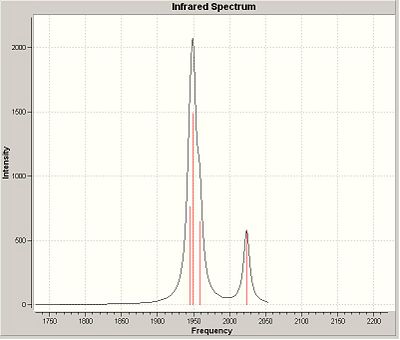 |

|
It can be noted that the the frequency chart of the CIS- isomer contains 4 peaks around the νCO region, whereas the TRANS- isomer contains but one.
This can be explained mainly due to the overall symmetry of the complex. The trans complex has greater symmetry than the cis complex, and this is shown by the fact that the νCO stretches are not degenerate, whereas the stretches in the trans complex are.
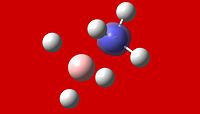
Exercise 3 - Mini-Project - Ammonia Borane NH3BH3 and its application as a Hydrogen Storage Medium
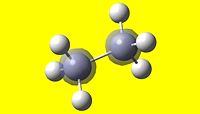
Ammonia Borane (pictured below), is being researched extensively as a new medium for the storage of Hydrogen, with the onset of hydrogen as a source of energy.
Intrinsically, it is isoelectronic with ethane, and shares a similar structure. As opposed to the non-polar bond nature of ethane, ammonia borane exists thanks to the dative bond between the boron and nitrogen. The lone pair present on the nitrogen in ammonia donates itself into the empty p orbital of the lewis-acidic boron, thus forming a dative bond.
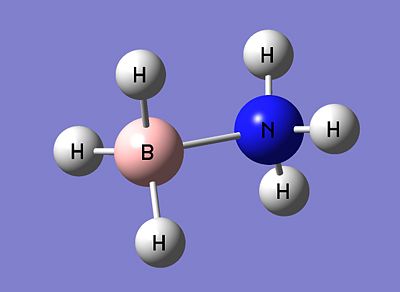 |
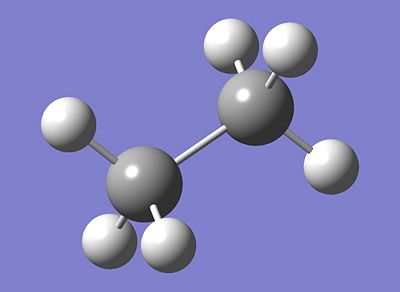
|
| Ammonia Borane, NH3BH3 | Ethane, C2H6 |
| Optimised Bonding Data | ||||
| Property | NH3BH3 | C2H6 | ||
| B-N/C-C Bond Length | 1.65 | 1.53 | ||
| H-X Bond Length (where X=C/B/N) | 1.21(B-H) | 1.02(N-H) | 1.09 | |
| Question | Ammonia Borane | Ethane |
| What is the file type? | .fch | .fch |
| What is the calculation type? | FOPT | SP |
| What is the calculation method? | RMP2-FC | RMP2-FC |
| What is the basis set? | 6-311G(D,P) | 6-311G(D,P) |
| What is the final energy in a.u. (atomic units)? | -86.96 | -79.57 |
| What is the gradient? | 0.0000798 | 0.0000000 |
| What is the dipole moment? (debye) | 5.56 | 0.00 |
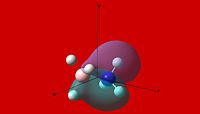
MO and NBO Analysis and Comparison of Ammonia Borane and Ethane
| Molecular Orbital Comparison | ||||
| NH3BH3 | ||||
| Molecular Orbital | ||||
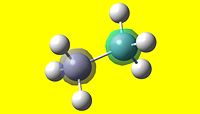
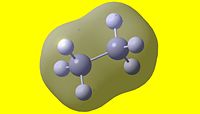
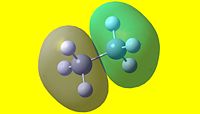
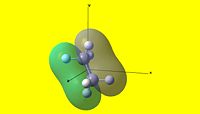
| 1 | 2 | 3 | 4 | 5 |
 |
 |
 |
 |
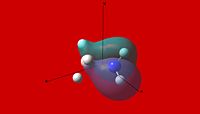
|
| C2H6 | ||||
 |
 |
 |
 |
|
| NH3BH3 | ||||
| Molecular Orbital | ||||
| 6 | 7 | 8 | 9 | 10 |
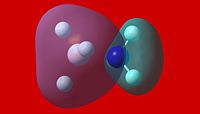 |
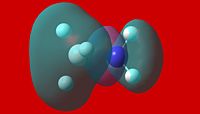 |
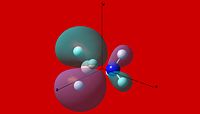 |
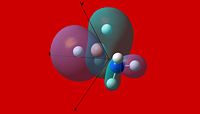 |

|
| C2H6 | ||||
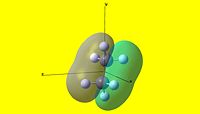 |
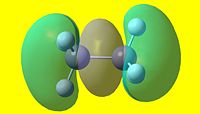 |
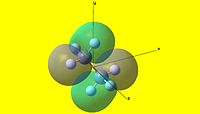 |
 |

|
| NBO Picture | |
| NH3BH3 | C2H6 |
 |
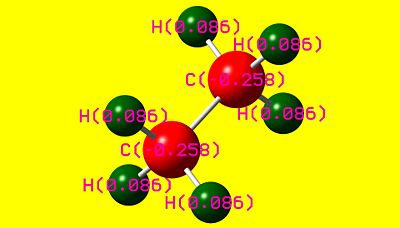
|
As can be seen, there are two striking differences between the two NBO pictures. The first is that charge distribution-wise, ethane is completely symmetric, whereas ammonia borane is rather polar. The second is that the hydrogens attached to the boron are not equivalent to the hydrogens attached to the nitrogen in ammonia borane. From this, it is apparent that ammonia borane is polar whereas homonuclear ethane is not. This has large consequences to the physical properties of each species, as will be discussed in the following section.
Characteristics of Ammonia Borane and its application as a hydrogen storage medium
It is interesting that while Ammonia Borane is isoelectronic, thus having the same number of electrons and structure, with ethane, their physical properties vary substantially. Ammonia Borane, for example, exists as a orthorhombic crystalline solid at room temperature, whereas ethane is a gas. Reportedly, the melting point for Ammonia Borane has a melting point of 104o, whereas that of ethane is -181.76o, differing greatly.
Analysing the values above concerning the final energies of each molecule, it can be seen that ammonia borane has the lower energy and is therefore more thermodynamically stable than its counterpart.
The reason behind this astonishing phenomenon is due to the highly polar nature of ammonia borane. Its crystalline structure is in fact the result of a specialised form of hydrogen bonding, thought by many formally as the interaction between an electronegative atom and hydrogen, which is deshielded into a protonic state. This special form of hydrogen bonding does not follow the convention of a hydrogen, atom, hydrogen, atom alternative pattern, but is rather an interaction between respective hydrogens bonded alternatively to boron and nitrogen. Due to the difference in electronegativity of the two atoms and thus the polarity of the B-N bond, the hydrogens are placed into different environments. The hydrogens on the boron are found to be rather hydridic, as visualised in the NBO colour picture as being slightly negatively charged. Conversely, the hydrogens situated on the nitrogen are acidic. As such, there is a potential δ+, δ- relationship between respective hydrogens.
This phenomenon is known as Dihydrogen Bonding[1] and also goes on to explain the varying B-H/N-H bond lengths. It is found that the N-H bond is rather flat in the expense of the B-H bond, which is bent.
Conclusion
Although ammonia borane is isoelectric with ethane, through the use of computational chemistry in the elucidation of its various properties, we have found that ammonia borane is in fact, incredibly more stable.
Ammonia is an excellent carrier of hydrogen due to its high hydrogen content (19.6 wt%, 0.145 kg L−1 H2)[2], as well as the fact it is a stable solid in the atmosphere, making it also easy to transport.