Rep:Mod:cb2811ICompMP
Ionic Liquids, designer solvents
"Ionic liquids" is the name given to salts that are in the liquid state at room temperature. This is an unusual characteristic, since salts, which are formed by oppositely charged cations linked together by ionic bonds, typically have a high boiling point due to the strong nature of the ionic bonds (e.g. NaCl has a b.p. of 801 oC). Thus, salts with a b.p. below 100 oC are considered ionic liquids.
Thanks to this particular property, ionic liquids have many interesting applications, such as very convenient electrolytes for electric batteries, due to their ability of increasing the battery life by drying slower than water, and also as "greener" solvent, due to their low vapour pressure which makes them less volatile.
Usually ionic liquids are composed of bulky and charge delocalized, asymmetric organic cations and inorganic anions. The charge delocalization over a large area of the molecule is the key factor to avoid strong ionic interactions between the ions, hence resulting in a typical ionic salt. The physical properties of these species can be tuned by modifying the constituents anions, for example by introducing functional groups or by experimenting different combinations of ions.
Computational chemistry represents a powerful tool for investigating ionic liquids at molecular level, thus providing a way to predict their physical properties before actually synthesize them in the lab.[1]
The aim of this project is to use computational analysis to first compare and contrast the properties of three tetramethyl 'onium' cations, i.e. [N(CH3)4]+, [P(CH3)4]+ and [S(CH3)3]+, and then investigate the influence of the addition of two different functional groups (one electron withdrawing group and an electron donating group) to the [N(CH3)4]+ cation.
Comparison of selected 'onium' cations
Optimization
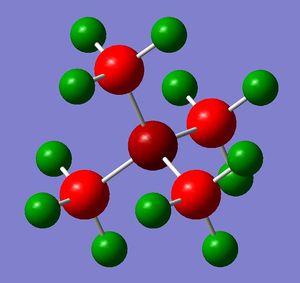
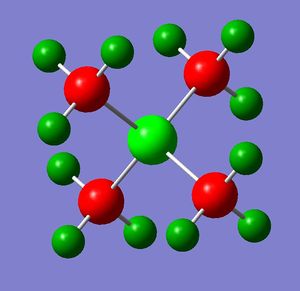
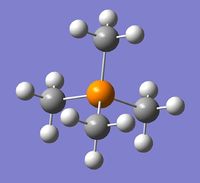
The optimizations of the geometry of [N(CH3)4]+, [P(CH3)4]+ and [S(CH3)3]+ were carried out using the full basis set 6-31G(d,p), B3LYP method and the keyword nonsymm was added to avoid symmetry constraints on the structures of the cations, hence providing a better optimization.
| Cation | [N(CH3)4]+ | [P(CH3)4]+ | [S(CH3)3]+ |
| Optimized geometry |  |
 |

|
| File Link | File:CB NCH34 OPT.txt | File:CB PCH34 OPT.txt | |
| File Type | .log | .log | .log |
| Calculation Type | FOPT | FOPT | FOPT |
| Calculation Method | RB3LYP | RB3LYP | RB3LYP |
| Basis Set | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) |
| Charge | 1 | 1 | 1 |
| Spin | Singlet | Singlet | Singlet |
| E(RB3LYP) / a.u. | -214.18126910 | -500.82701391 | -517.68326631 |
| RMS Gradient Norm / a.u. | 0.00003217 | 0.00009884 | 0.00007309 |
| Imaginary Freq | - | - | - |
| Dipole Moment / Debye | 0.0002 | 0.0008 | 0.9654 |
| Point Group | C1 | C1 | C1 |
| Job cpu time | 0 days 0 hours 5 minutes 50.2 seconds | 0 days 0 hours 6 minutes 36.8 seconds | 0 days 0 hours 4 minutes 27.1 seconds |
| D-Space Link | DOI:10042/27652 | DOI:10042/27650 | DOI:10042/27651 |
| Item | Item Value Threshold Converged?
Maximum Force 0.000089 0.000450 YES
RMS Force 0.000026 0.000300 YES
Maximum Displacement 0.001694 0.001800 YES
RMS Displacement 0.000418 0.001200 YES
Predicted change in Energy=-1.707019D-07
Optimization completed.
-- Stationary point found.
|
Item Value Threshold Converged?
Maximum Force 0.000290 0.000450 YES
RMS Force 0.000061 0.000300 YES
Maximum Displacement 0.001497 0.001800 YES
RMS Displacement 0.000485 0.001200 YES
Predicted change in Energy=-8.136618D-07
Optimization completed.
-- Stationary point found.
|
Item Value Threshold Converged?
Maximum Force 0.000166 0.000450 YES
RMS Force 0.000056 0.000300 YES
Maximum Displacement 0.000870 0.001800 YES
RMS Displacement 0.000328 0.001200 YES
Predicted change in Energy=-2.668752D-07
Optimization completed.
-- Stationary point found.
|
| [N(CH3)4]+ | [P(CH3)4]+ | [S(CH3)3]+ | |
|---|---|---|---|
| X-C (Å) | 1.51 Lit. 1.451[2] |
1.82 Lit. 1.841[3] |
1.82 Lit. 1.83[4] |
| C-H (Å) | 1.09 | 1.09 | 1.09 |
| C-X-C (o) | 109.5 | 109.5 | 102.7 |
| H-C-X (o) | 108.9 | 109.9 | 111.1 |
| H-C-H (o) | 110.0 | 109.0 | 109.4 |
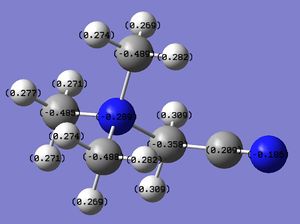
The first comparison between the three cations involves their structures. All the cations have a tetrahedral geometry (i.e. central atom is sp3 hydridised) but, while N and P atoms are bonded to 4 methyl groups, S is bonded to 3 methyl groups and it has a lone pair in the empty sp3 orbital.
By inspection of Table 1 it is possible to see that, while C-H bond is the same for the three cations, X-C bond is shorter in [N(CH3)4]+ than in [P(CH3)4]+ and [S(CH3)3]+. This can be explained by considering that N is more electronegative (Δe=3.0) than P (Δe=2.1) and S (Δe=2.5), therefore pulls the electrondensity of C more stronly than P and S, resulting in a shorter bond. Furthermore, N and C are in the same row of the PT while P and S are one row below, thus N-C orbital overlap is better compared to P-C and S-C, yielding a stronger (and shorter) bond. The X-C values show a good agreement with the literature, demonstarting that good optimizations of the structures were obtained.
By comapring the C-X-C bond angles, one can see that C-N-C and C-P-C are the same but they are larger than C-S-C. This is rationalised by considering that S is in the 6th group of the PT while N and P are in the 5th, therefore the S atom has one lone pair of electrons in the empty sp3 orbital, which repels the methyl groups close to each other, determining C-S-C bond angles of 102.7o, i.e. smaller than the ideal tetrahedral geometry (i.e. 109o C-X-C angles). Furthermore, by looking at the bond orbital contributions of the S-C bonds it can be observed that the S orbitals are not exactly sp3 hybridised. In fact, for sp3 hybridisation s and p orbitals contributions are expected to be 25% and 75%, respectively, but the population analysis of [S(CH3)3]+ shows orbital contributions of 17% for the s orbital and 82% for p orbitals. Therefore, the S orbitals appear to be more p-like. Considering that p orbitals are typically aligned orthogonal to each other, this would explain the C-S-C bond angles being in between 90o and 109o.
BD ( 1) C 5 - S 13 ( 48.67%) 0.6976* C 5 s( 19.70%)p 4.07( 80.16%)d 0.01( 0.14%) ( 51.33%) 0.7165* S 13 s( 16.95%)p 4.86( 82.42%)d 0.04( 0.63%)
Frequency analysis
A frequency analysis has been carried out for each cation using the B3LYP method and full basis set 6-31G(d,p), in order to confirm that a minimum was achieved for the structure of each cation.
| Cation | [N(CH3)4]+ | [P(CH3)4]+ | [S(CH3)3]+ |
| File Link | File:CB NCH34 FREQ.txt | File:CB PCH34 FREQ.txt | |
| File Type | .log | .log | .log |
| Calculation Type | FREQ | FREQ | FREQ |
| Calculation Method | RB3LYP | RB3LYP | RB3LYP |
| Basis Set | 6-31G(d,p) | 6-31G(d,p) | 6-31G(d,p) |
| Charge | 1 | 1 | 1 |
| Spin | Singlet | Singlet | Singlet |
| E(RB3LYP) / a.u. | -214.18126894 | -500.82701385 | -517.68326635 |
| RMS Gradient Norm / a.u. | 0.00003183 | 0.00009877 | 0.00007303 |
| Imaginary Freq | - | - | - |
| Dipole Moment / Debye | 0.0002 | 0.0008 | 0.9654 |
| Point Group | C1 | C1 | C1 |
| Job cpu time | 0 days 0 hours 8 minutes 1.1 seconds | 0 days 0 hours 8 minutes 45.7 seconds | 0 days 0 hours 3 minutes 48.6 seconds |
| D-Space Link | DOI:10042/27657 | DOI:10042/27659 | DOI:10042/27660 |
| Low Frequencies / cm-1 | Low frequencies --- -16.8417 -13.8185 -0.0006 0.0005 0.0011 5.3463 Low frequencies --- 182.3169 283.9279 288.6587 |
Low frequencies --- -15.6924 -0.0018 0.0026 0.0027 16.9158 20.2183 Low frequencies --- 154.7174 186.7327 191.8493 |
Low frequencies --- -26.7862 -0.0042 -0.0019 -0.0017 6.3790 11.6698 Low frequencies --- 158.7323 193.5518 200.7451 |
| IR Spectrum | 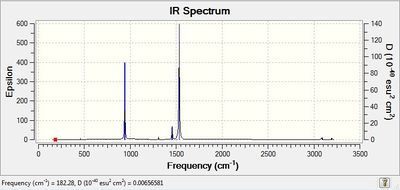 |
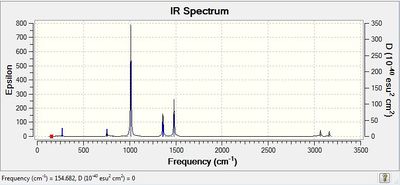 |
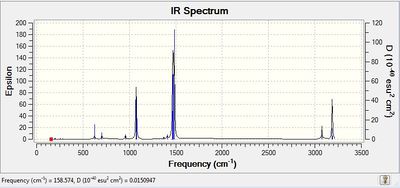
|
The Low Frequencies lie in the range +/- 30 cm-1 for all the three cations, indicating that the basis set used is not very good and some soft motions (rotation/translation) of the central atom are present. However, as it can be seen from the spectra of the 3 cations(and also from their vibrational frequencies reported in their txt files), all the frequencies are positive, thus indicating that a minimum was reached for each cation structure.
Population Analysis
The energy of the cations was calculated using the B3LYP method, full basis set 6-31G(d,p) and the keyword pop=(nbo, full) was added in order to activate the MO and NBO analyses of the cations.
MOs analysis
| [N(CH3)4]+ | [P(CH3)4]+ | [S(CH3)3]+ | |
|---|---|---|---|
| File Link | File:CB NCH34 MOs.txt | File:CB PCH34 MOs.txt | |
| D-Space Link | DOI:10042/27661 | DOI:10042/27662 | DOI:10042/27663 |
An accurate description of the interactions occurring in 5 MOs of [N(CH3)4]+, ranging from highly bonding to highly antibonding, has been provided in Table 2.
NBO analysis
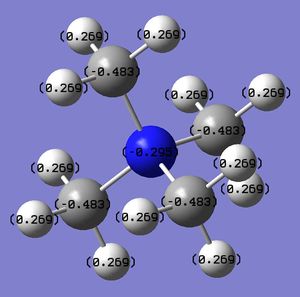
A Natural Bonding Orbital (NBO) analysis has been carried out in order to compare and contrast the charge distribution of the three cations, as reported in Table 3.
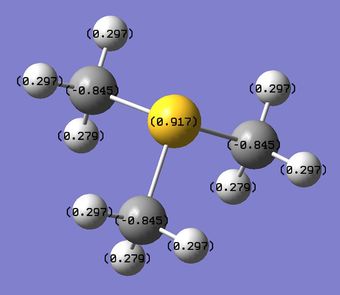
By analysing the charge distribution in each cation, we can see that the central atoms N, P and S are all electron deficient (since they donate one electron pair to form a bond with C), however, N is negatively charged while P and S are positively charged. This can be explained by considering that N has a better orbital overlap with C (because they are in the same row of the PT, thus the orbitals are of similar size) and it is more electronegative than P and S, therefore its strong inductive effect allows it to maintain a negative charge. On the other hand, P and S do not have this inductive effect, since they have larger orbitals, which do not overlap very well with C AOs, and they also have lower (P) and similar(S) electronegativity than C. Thus, P and S are positively charged due to the electron pair donation (S is less positively charged than P because it is slightly more electronegative, i.e. S Δe=2.5, P Δe=2.1).
In order to balance the positive charge of the heteroatoms, the C atoms are negatively charged thanks to the withdrawing of electron density from the H atoms. As expected, the amount of negative charge on C atoms is proportional to the amount of positive charge on the heteroatom of the cation, hence the C in [P(CH3)4]+ have the highest charge value, followed by the C in [S(CH3)3]+ and finally those in [S(CH3)4]+. All the H atoms in the three cations remain positively charged, to give an overall charge of the cations of approximately 1+.
| [N(CH3)4]+ | BD ( 1) C 5 - N 17
( 33.65%) 0.5801* C 5 s( 20.78%)p 3.81( 79.06%)d 0.01( 0.16%)
( 66.35%) 0.8146* N 17 s( 25.00%)p 3.00( 74.97%)d 0.00( 0.03%)
BD ( 1) C 9 - H 10
( 63.47%) 0.7967* C 9 s( 26.42%)p 2.78( 73.53%)d 0.00( 0.05%)
( 36.53%) 0.6044* H 10 s( 99.95%)p 0.00( 0.05%)
|
| [P(CH3)4]+ | BD ( 1) C 5 - P 17
( 59.56%) 0.7717* C 5 s( 25.21%)p 2.96( 74.71%)d 0.00( 0.08%)
( 40.44%) 0.6359* P 17 s( 25.00%)p 2.97( 74.15%)d 0.03( 0.85%)
BD ( 1) C 9 - H 10
( 64.78%) 0.8049* C 9 s( 24.89%)p 3.02( 75.06%)d 0.00( 0.04%)
( 35.22%) 0.5934* H 10 s( 99.95%)p 0.00( 0.05%)
|
| [S(CH3)3]+ | BD ( 1) C 5 - S 13
( 48.67%) 0.6976* C 5 s( 19.70%)p 4.07( 80.16%)d 0.01( 0.14%)
( 51.33%) 0.7165* S 13 s( 16.95%)p 4.86( 82.42%)d 0.04( 0.63%)
BD ( 1) C 9 - H 10
( 64.83%) 0.8052* C 9 s( 26.50%)p 2.77( 73.45%)d 0.00( 0.05%)
( 35.17%) 0.5931* H 10 s( 99.95%)p 0.00( 0.05%)
|
The analysis of the relative orbitals contribution of the C and heteroatom to the C-X bond, reported in Table 4, shows that N contributes more than 50% to the N-C bond, S and C contributes almost the same, i.e. 50% each, to the S-C bond and P contributes less than C in the P-C bond. This reflects the influence of relative electronegativity of the atoms on the charge distribution, as discussed above. In fact, N, which has the highest electronegativity (Δe=3.0) contributes the most to the N-C bond cause it holds electron density close to itself; S, which has the same electronegativity as carbon (Δe=2.5), has an equal contribution to the S-C bond as the C atom; P, instead, has a lower contribution to the P-C bond because it has a lower electronegativity (Δe=2.1) than C.
The traditional picture of [N(CH3)4]+ showing the "formal" positive charge on the N atom represents the electron deficiency of N due to its electron pair donation to form the N-C bond, rather than the actual charge on the atom, which remains negative, as we have seen above in the charge distribution analysis. In fact, the actual positive charge of the cation is located on the 12 H atoms. However, since it would be difficult (and not practical) drawing the "formal" positive charge on each one of the 12 H atoms, the traditional representation of the charge on the N is adopted. A better representation of the cation would be the molecular structure enclosed in squared brakets with the positive charge in superscript outside the braket, as shown here. 
Influence of functional groups
The effects of adding an EWG (CN) and an EDG (OH) to the [N(CH3)4]+ cation were investigated. The optimization of the geometry and a frequency analysis of [N(CH3)3(CH2OH)]+ and [N(CH3)3(CH2CN)]+ were carried out to check the attainment of a minimum for each structure. Then, the MO and NBO analyses of the two species have been carried out to compare and contrast the different MOs interactions and charge distribution. Finally, the HOMO and the LUMO of [N(CH3)4]+, [N(CH3)3(CH2OH)]+ and [N(CH3)3(CH2CN)]+ were compared and contrasted in order to obtain information about the energy change of these MOs and, hence, predict some physical properties of the cations.
Optimization
The optimizations of the geometry of [N(CH3)3(CH2OH)]+ and [N(CH3)3(CH2CN)]+ were carried out using the full basis set 6-31G(d,p), B3LYP method and the keyword nonsymm was added to avoid symmetry constraints on the structures of the cations, hence providing a better optimization.
| Cation | [N(CH3)3(CH2OH)]+ | [N(CH3)3(CH2CN)]+ |
| Optimized geometry | 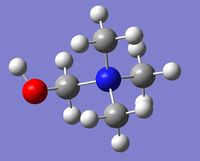 |
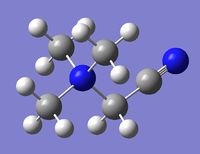
|
| File Link | File:CB NOH OPT.txt | File:CB NCN OPT.txt |
| File Type | .log | .log |
| Calculation Type | FOPT | FOPT |
| Calculation Method | RB3LYP | RB3LYP |
| Basis Set | 6-31G(d,p) | 6-31G(d,p) |
| Charge | 1 | 1 |
| Spin | Singlet | Singlet |
| E(RB3LYP) / a.u. | -289.39471437 | -306.39376906 |
| RMS Gradient Norm / a.u. | 0.00000872 | 0.00006410 |
| Imaginary Freq | - | - |
| Dipole Moment / Debye | 2.1352 | 5.7648 |
| Point Group | C1 | C1 |
| Job cpu time | 0 days 0 hours 13 minutes 7.7 seconds | 0 days 0 hours 18 minutes 37.6 seconds |
| D-Space Link | DOI:10042/27728 | DOI:10042/27729 |
| Item | Item Value Threshold Converged?
Maximum Force 0.000017 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000917 0.001800 YES
RMS Displacement 0.000250 0.001200 YES
Predicted change in Energy=-2.298550D-08
Optimization completed.
-- Stationary point found.
|
Item Value Threshold Converged?
Maximum Force 0.000037 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000300 0.001800 YES
RMS Displacement 0.000070 0.001200 YES
Predicted change in Energy=-3.498828D-09
Optimization completed.
-- Stationary point found.
|
Frequency Analysis
A frequency analysis has been carried out for each cation using the B3LYP method and full basis set 6-31G(d,p), in order to confirm that a minimum was achieved for the structure of each cation.
| Cation | [N(CH3)3(CH2OH)]+ | [N(CH3)3(CH2CN)]+ |
| File Link | File:CB NOH FREQ.txt | File:CB NCN FREQ.txt |
| File Type | .log | .log |
| Calculation Type | FREQ | FREQ |
| Calculation Method | RB3LYP | RB3LYP |
| Basis Set | 6-31G(d,p) | 6-31G(d,p) |
| Charge | 1 | 1 |
| Spin | Singlet | Singlet |
| E(RB3LYP) / a.u. | -289.39471437 | -306.39376906 |
| RMS Gradient Norm / a.u. | 0.00000867 | 0.00006413 |
| Imaginary Freq | - | - |
| Dipole Moment / Debye | 2.1351 | 5.7648 |
| Point Group | C1 | C1 |
| Job cpu time | 0 days 0 hours 11 minutes 31.2 seconds | 0 days 0 hours 11 minutes 24.1 seconds |
| D-Space Link | DOI:10042/27734 | DOI:10042/27735 |
| Low Frequencies / cm-1 | Low frequencies --- -11.7502 -0.0008 -0.0004 0.0006 8.3588 14.4115 Low frequencies --- 134.0656 214.0466 255.7636 |
Low frequencies --- -7.8225 0.0000 0.0005 0.0008 5.8158 17.0094 Low frequencies --- 91.9350 154.4722 212.7851 |
| IR Spectrum |  |
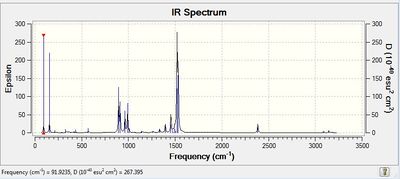
|
Population Analysis
The energy of the cations was calculated using the B3LYP method, full basis set 6-31G(d,p) and the keyword pop=(nbo, full) was added in order to activate the MO and NBO analyses of the cations.
MOs Analysis
| [N(CH3)3(CH2OH)]+ | [N(CH3)3(CH2CN)]+ | |
|---|---|---|
| File Link | File:CB NOH MOs.txt | File:CB NCN MOs.txt |
| D-Space Link | DOI:10042/27737 | DOI:10042/27738 |
NBO Analysis
A Natural Bonding Orbital (NBO) analysis has been carried out in order to compare and contrast the charge distribution and the bond orbitals contributions of the two cations, as reported in Table 5 and Table 6.
| [N(CH3)3(CH2OH)]+ | BD ( 1) C 1 - N 16
( 34.06%) 0.5836* C 1 s( 20.82%)p 3.80( 79.02%)d 0.01( 0.16%)
( 65.94%) 0.8120* N 16 s( 25.48%)p 2.92( 74.49%)d 0.00( 0.03%)
BD ( 1) C 5 - N 16
( 32.72%) 0.5720* C 5 s( 20.27%)p 3.92( 79.55%)d 0.01( 0.18%)
( 67.28%) 0.8202* N 16 s( 23.46%)p 3.26( 76.51%)d 0.00( 0.03%)
BD ( 1) C 5 - O 17
( 33.90%) 0.5822* C 5 s( 23.71%)p 3.21( 76.04%)d 0.01( 0.24%)
( 66.10%) 0.8130* O 17 s( 32.28%)p 2.10( 67.64%)d 0.00( 0.08%)
BD ( 1) O 17 - H 18
( 76.27%) 0.8733* O 17 s( 22.10%)p 3.52( 77.83%)d 0.00( 0.07%)
( 23.73%) 0.4872* H 18 s( 99.77%)p 0.00( 0.23%)
Donor NBO Acceptor NBO E(2) Ej-Ei F(i,j)
(kcal/mol) (a.u.) (a.u.)
LP (2) O 17 /140. BD*(1) C5 - N16 18.95 0.51 0.088
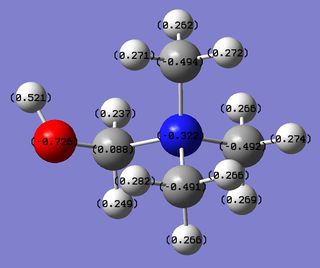
The introduction of the electron donating OH group generates a disruption in the symmetry of the cation, which in turn leads to a different charge distribution between the atoms, compared to [N(CH3)4]+ cation.First of all, as in [N(CH3)4]+ cation, N(16) is negatively charged and it has the highest orbital contribution to the C(1)-N(16) bond due to the greater electronegativity of N compared to C. The same observation holds true for C(8)-N(16) and C(12)-N(16) bonds. Then, the higher contribution of N(16) to the C(5)-N(16) bond is a consequence of the presence of the neighbouring OH group. In fact, the highly electronegative O(17) pulls electron density away from C(5) allowing N(16), which normally is slightly more electronegative than C, to withdrawn electron density away from C(5) more easily than from C(1), C(8) and C(12). As a consequence of this effect, C(5) is the only positively charged C. As already mentioned, the higher electronegativity of O compared to C and H atoms explains both the greatest contribution of O(17) to the O(17)-C(5) and O(17)-H(18) bonds, and also the fact that O(17) is the most negatively and H(18) the most positively charged atom in the cation. All the other H atoms are positively charged due to the good C-H orbitals overlap that allows C (which is more electronegative than H) to withdrawn the electron density away from the H atoms. As a result, C(1), C(8) and C(12) are negatively charged. Fianlly, it is interesting to note the presence of a stereoelectronic interaction (second order NBO) originated from the O(17) LP donation into the C(5)-N(16) antibonding σ* MO, which has the effect of weakening the C(5)-N(16) and providing partial delocalization. |
| [N(CH3)3(CH2CN)]+ | BD ( 1) C 1 - N 16
( 33.12%) 0.5755* C 1 s( 20.23%)p 3.94( 79.60%)d 0.01( 0.17%)
( 66.88%) 0.8178* N 16 s( 25.36%)p 2.94( 74.61%)d 0.00( 0.03%)
BD ( 1) C 5 - N 16
( 35.48%) 0.5956* C 5 s( 20.81%)p 3.80( 79.05%)d 0.01( 0.14%)
( 64.52%) 0.8033* N 16 s( 24.07%)p 3.15( 75.89%)d 0.00( 0.04%)
BD ( 1) C 5 - C 17
( 51.35%) 0.7166* C 5 s( 27.00%)p 2.70( 72.93%)d 0.00( 0.07%)
( 48.65%) 0.6975* C 17 s( 52.03%)p 0.92( 47.93%)d 0.00( 0.04%)
BD ( 1) C 17 - N 18
( 42.68%) 0.6533* C 17 s( 47.95%)p 1.09( 52.03%)d 0.00( 0.02%)
( 57.32%) 0.7571* N 18 s( 45.15%)p 1.21( 54.49%)d 0.01( 0.36%)
BD ( 2) C 17 - N 18
( 47.13%) 0.6865* C 17 s( 0.00%)p 1.00( 99.94%)d 0.00( 0.05%)
( 52.87%) 0.7271* N 18 s( 0.00%)p 1.00( 99.59%)d 0.00( 0.41%)
BD ( 3) C 17 - N 18
( 49.36%) 0.7026* C 17 s( 0.09%)p99.99( 99.87%)d 0.46( 0.04%)
( 50.64%) 0.7116* N 18 s( 0.03%)p99.99( 99.57%)d15.00( 0.40%)
Donor NBO Acceptor NBO E(2) Ej-Ei F(i,j)
(kcal/mol) (a.u.) (a.u.)
LP (1) N 18 /122. RY*(1) C17 16.69 1.20 0.127
LP (1) N 18 /149. BD*(1) C5 - C17 12.71 0.94 0.098
The introduction of the electron withdrawing CN group generates a disruption in the symmetry of the cation, which in turn leads to a different charge distribution between the atoms, compared to [N(CH3)4]+ cation.First of all, as in [N(CH3)4]+ cation, N(16) is negatively charged and it has the highest orbital contribution to the C(1)-N(16) bond due to the greater electronegativity of N compared to C. The same observation holds true for C(5)-N(16), C(8)-N(16) and C(12)-N(16) bonds. However, in C(5)-N(16) bond N(16) provides a slightly lower contribution than in the other C-N bonds; this is probably a consequence of the presence of the neighbouring electron withdrawing CN group. In the C(5)-C(17) bond, C(5) has a slightly higher contribution than C(17), as a consequence of the less electron density present on C(17), which is withdrawn by the N(18). This is reflected in the C(17) being positively charged, while all the other C atoms are negatively charged (due to the electron density withdrawning from the H atoms). However, C(5) is still slightly less negatively charged than C(8) and C(12) and this is because it is bonded to a lower number of H atoms from which it can withdrawn electron density. As a consequence, the 2 H atoms to which C(5) is bounded, i.e. H(7) and H(9), are more positively charged than the other H atoms due to the greater electron sucking from C(5), which tries to compensate its electron deficiency. Then, in the three C(17)-N(18) bonds the highest contribution is given by N(18), due to its higher electronegativity than C. Moreover, all the H atoms are positively charged due to the good C-H orbitals overlap that allows C (which is more electronegative than H) to withdrawn the electron density away from the H atoms. Fianlly, the presence of a second order NBO interaction involving the N(18) LP donation into the antibonding C(5)-C(17) σ* MO is quite surprising considering that N(18) LP is aligned periplanar to the C(5)-C(17) σ* MO, thus such stereoelectronic interaction would be unexpected. The effects of this interaction are the weakening of the C(5)-C(17) bond and the partial electrons delocalization over the C(5),C(17) and N(18) atoms. |
Comparison between [N(CH3)4]+, [N(CH3)3(CH2OH)]+ and [N(CH3)3(CH2CN)]+
By comparing the shape of the HOMO of [N(CH3)4]+, [N(CH3)3(CH2OH)]+ and [N(CH3)3(CH2CN)]+ the following observation are made:
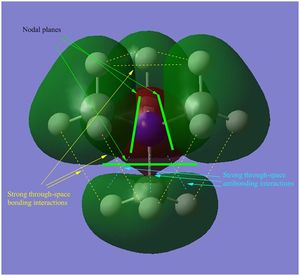
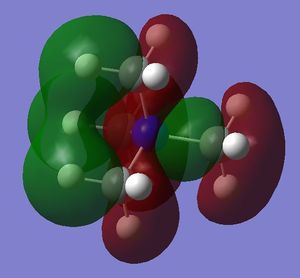
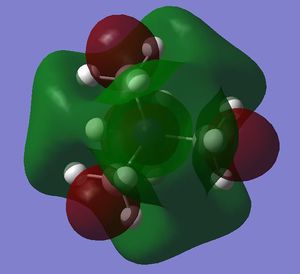
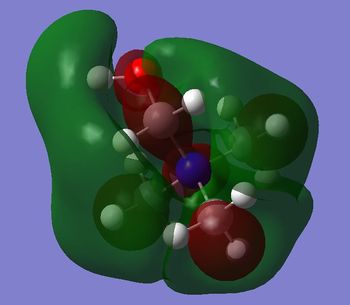
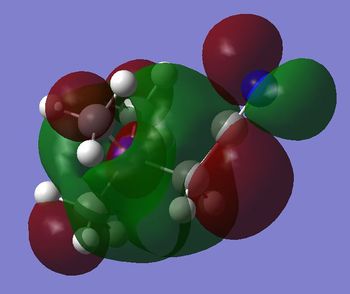
- in [N(CH3)4]+ there are weak bonding AO interactions, through-space weak bonding (between red-red and green-green surfaces) and strong antibonding (between red-green adjacent surfaces) interactions, the MO is partly delocalised and there are 5 nodal planes at C and N atoms. Overall, the MO is weakly bonding.
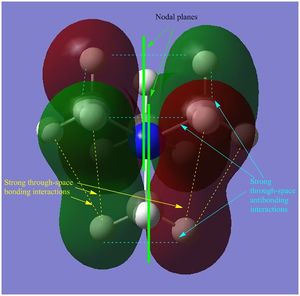
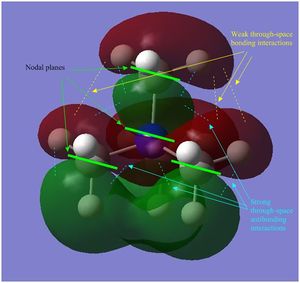
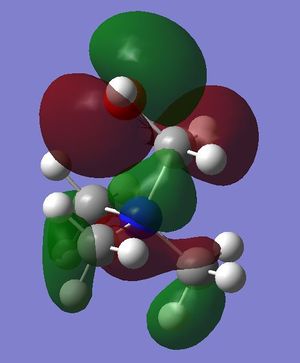
- in [N(CH3)3(CH2OH)]+ mainly antibonding through-space interactions are observed throughout the whole molecule, the only bonding interactions are represented by the weak through-space bonding overlap between the H atoms on the methyl groups and also the in-phase orbital overlap between the oxygen LP and the C p orbital and H s orbital of the CH2 unit. Apart from over the O-CH2, unit there is almost no delocalisation, and there are 6 nodal planes, one on each non-H atom.
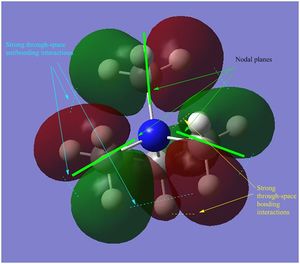
- the HOMO in [N(CH3)3(CH2CN)]+ is almost entirely non-bonding, apart from the through-space π antibonding interaction between the FO (fragment orbital) of the CN unit and the FO of the CH2 unit. However, the HOMO is slightly bonding probably due to the large bonding interaction within the nitrile unit (i.e. π bonding FO generated from the C p and N p AOs overlap), which overall stabilizes the cation.
These observations provide a rational explanation behind the order of stability of the HOMO of the three cation, which is [N(CH3)4]+>[N(CH3)3(CH2CN)]+>[N(CH3)3(CH2OH)]+ (going from the HOMO with the lowest energy, i.e. the most stable, to the one with the highest energy). This means that by adding the electron withdrawing CN group the HOMO of the [N(CH3)4]+ cation is destabilized of about 210 kJmol-1, and upon addition of the electron donating OH group it is destabilized even further, i.e. ~250 kJmol-1.
By comparing the shape of the LUMOs of the three cations the following observation are made:
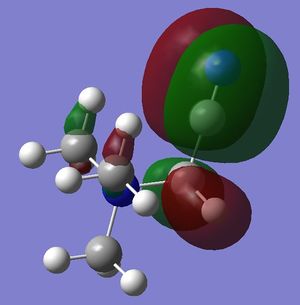
- in [N(CH3)4]+ there are strong through-space antibonding interactions (between red-green adjacent surfaces) and 4 nodal planes in the internuclear region of each C-N bond. Weak through space bonding interactions between the H atoms of adjacent methyl groups are present; however, these are not strong enough to confer an overall bonding character to the LUMO, which remains antibonding.
- in [N(CH3)3(CH2OH)]+ mainly strong antibonding through-space interactions are observed; in fact, in addition to the same antibonding interactions observed also in the LUMO of [N(CH3)4]+, here there is also a strong antibonding interaction between the in-phase orbitals overlap of the N-CH2-O unit (red central surface) and the surrounding orbitals (green surfaces).
- in [N(CH3)3(CH2CN)]+ there is a favourable in-phase orbitals overlap between the nitrile C and the CH2 carbon, resulting in a π-bonding interaction, which in turn overlap favourably with the orbitals of the other C atoms (green surfaces). However, the LUMO remains overall antibonding due to the presence of the same strong antibonding interactions also present in the other 2 cations (between red-green adjacent surfaces in the central part of the molecule) and also due to the through-space antibonding interactions within the nitrile unit.
These observations provide a rational explanation behind the order of stability of the LUMO of the three cation, which is [N(CH3)3(CH2CN)]+>[N(CH3)4]+>[N(CH3)3(CH2OH)]+ (going from the LUMO with the lowest energy, i.e. the most stable, to the one with the highest energy). This means that by adding the electron withdrawing CN group the LUMO of the [[N(CH3)4]+ cation is stabilized of about 128 kJmol-1, while upon addition of the electron donating OH group it is destabilized of ~22 kJmol-1.
By looking at the chanage in HOMO-LUMO gap upon addition of functional groups to the [N(CH3)4]+ cation it can be noticed that:
- the energy gap reduces of ~220 kJmol-1 by adding the electron donating OH group;
- the energy gap reduces of ~500 kJmol-1 by adding the electron withdrawing CN group.
Considering that the smaller the HOMO-LUMO gap, the larger the degree of conjugation of the molecule, we can say that the addition of functional groups to the [N(CH3)4]+ cation has the effect of increasing its ability of charge delocatisation.
Conclusions
In conclusion, the presence of the CN group makes the [N(CH3)3(CH2CN)]+ cation a better electron acceptor (since the LUMO is lowered), while the presence of the alcohol group renders more difficult the acceptance of electrons (due to the LUMO being higher in energy) and theoretically easier the donation of electrons (higher HOMO). However, the potential ease of electron donation of [N(CH3)3(CH2OH)]+ is limited by the fact that the species is still a cation, thus it does not donate electrons. As a consequence, the higher acceptor ability of the [N(CH3)3(CH2CN)]+ will allow it to interact more strongly with the anions, thus increasing its boiling point compared to [N(CH3)4]+. On the other hand, the weaker acceptor ability of [N(CH3)3(CH2OH)]+ will result in weaker interactions with the anions, thus yielding a lower boiling point than [N(CH3)4]+.
References
<references>
- ↑ P. Hunt, 'Ionic liquids, designer solvents', 3rd Year Computational Lab, Imperial College, 2013
- ↑ J.E. Wollrab and V.W. Laurie, J. Chem. Phys., 1969, 51, 1583 DOI:10.1063/1.1672214
- ↑ D.R. Lide and D.E. Mann, J. Chem. Phys., 2004, 29, 914 DOI:10.1063/1.1744611
- ↑ D.E. Zuccaro and J.D. McCullough, Zeitschirft für Kristallog., 2010, 112, 401 DOI:10.1524/zkri.1959.112.1-6.401