Rep:Mod:caw116
BH3
B3LYP/6-31G
Summary of computed values
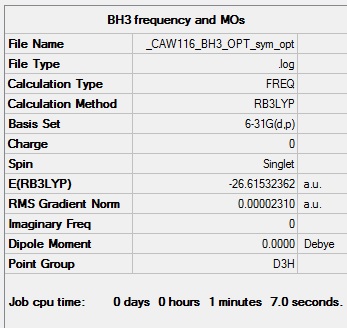
Item table
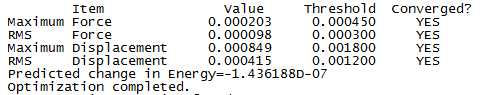
Frequency analysis

Jmol
BH3 |
Vibrations
| Mode | Frequency in cm-1 | Intensity | Type | Activity |
|---|---|---|---|---|
| 1 | 1163 | 93 | symmetrical bend | Yes |
| 2 | 1213 | 14 | symmetrical bend | Yes |
| 3 | 1213 | 14 | asymmetrical bend | Yes |
| 4 | 2582 | 0 | symmetrical stretch | No |
| 5 | 2715 | 126 | asymmetrical stretch | Yes |
| 6 | 2715 | 126 | symmetrical stretch | Yes |
Corresponding IR-spectrum

From the table it can be seen that the molecule exhibits 6 vibrational modes, however, mode 4 does not show a change in dipole moment, which makes it IR-unactive.
Smf115 (talk) 17:21, 23 May 2018 (BST)Correct assignment of the modes however, the symmetry assignment is missing and the effect of the degenerate modes on the number of peaks seen should be considered.
MO-diagram of BH3

Based on: Dr. Patricia Hunt, 2018, Imperial College London, "Lecture 4 Tutorial Problem Model Answers", p. 2
The computed MO's largely resemble the theoretical MO's, however, the computed ones show a greater degree of overlap and diffusion than the theoretical ones. This may mainly be due to the theoretical ones being supposed to be clear and easily readable, so that the computed ones are actually closer to reality. Qualitative MO theory still shows great usefulness and accuracy when it comes to predicting the appearance of MO's.
NH3
B3LYP/6-31G
Summary of computed values
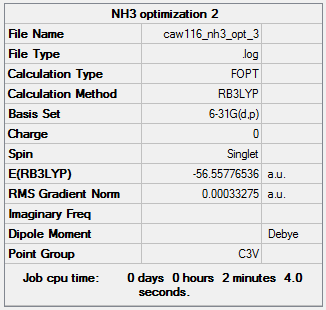
Item table

Frequency analysis

Jmol
NH3 |
BH3NH3
B3LYP/6-31G
Summary of computed values
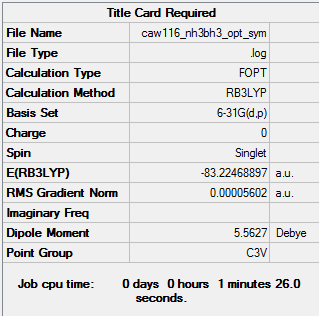
Item table

Frequency analysis

Jmol
NH3BH3 |
Energy analysis
E(BH3)= -26.61532362 a.u. E(NH3)= -56.55776536 a.u. E(BH3NH3)= -83.22468897 a.u.
Association energy AE = E(BH3NH3) - (E(NH3)+E(BH3))
= -83.22468897 a.u. -(-56.55776536 a.u.+-26.61532362 a.u.)
= - 0.05159999 a.u.
= -135.4499738 kJ/mol
The dissociation energy corresponds to the bond strength and is a positive value so
Bond strength = 135.4499738 kJ/mol
This bond strength is relatively low, compared to other organic molecules like methane or ammonia, whose bond strength lies above 400 kJ/mol.
Smf115 (talk) 17:23, 23 May 2018 (BST)Correct calculation method but consideration needs to be given to the accuracy of the final figures being reported. A relevant comparison is mentioned, however a reference should be used for the literature value.
BBr3
B3LYO/6-31G(d,p)LANL2DZ
Summary of computed values
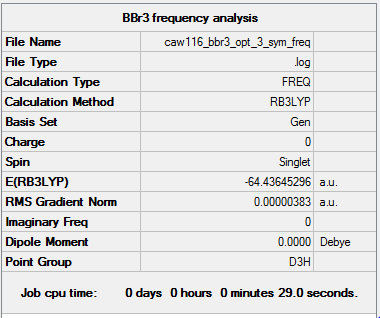
Item table

Frequency analysis

Jmol
BBr3 |
Project: Aromaticity
Benzene
B3LYP/6-31G
Summary of computed values
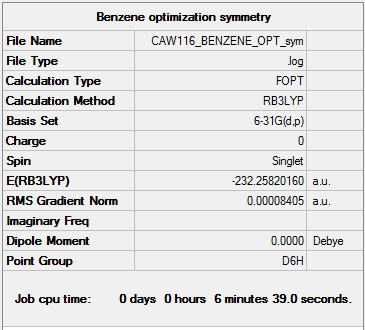
Item table

Frequency analysis

Jmol
Benzene |
Borazine
B3LYP/6-31G
Summary of computed values
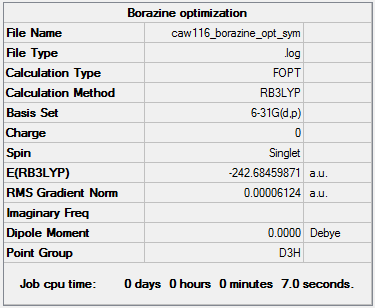
Item table

Frequency analysis

Jmol
Borazine |
NBO charge analysis


In benzene the charges are equally distributed throughout the molecule, with each carbon carrying a charge of -0.239 NBO charges and each hydrogen carrying a charge of 0.239 NBO charges. This is due to the carbon having a higher electronegativity value (2.5) than hydrogen (2.1). Thus, carbon pulls the electron closer than hydrogen.
In contrast to benzene, the charge in borazine is not as equally distributed. Here, each nitrogen carries are charge of -1.102 NBO charges, each boron a charge of 0.747 NBO charges, each hydrogen bound to a nitrogen a charge of -0.077 NBO charges and each hydrogen bound to boron a charge of 0.432 NBO charges. This can again be traced back to the difference in electronegativity of the different atoms. Boron has the lowest electronegativity (2.0), so it carries a partial positive charge and the adjacent hydrogen a small partial negative charge. Nitrogen has a very high electronegativity value (3.0) so that it carries the highest partial negative charge. Accordingly, the adjacent hydrogens carry a relatively high partial positive charge.
Smf115 (talk) 17:25, 23 May 2018 (BST)Nice explaination of the charges arising from the electronegativities of the atoms. To improve, other aspects such as symmetry and the overall charge shouls also be considered and the charge distribution on the moelcules should have the same colour range.
MO comparison
The concept of aromaticity
Aromatic compounds play an important role in biochemistry as well as industry. In general, aromatic compounds ought to obey Huckel's rule to be called aromatic. This rule states that "planar, fully conjugated and monocyclic systems", which have 4n+2 pi electrons in the valence bonding MO's, are called aromatic. [J. Clayden et al, 2012, Oxford University Press, "Organic Chemistry - Second edition", p.161] However, there have been discovered some examples in which the compound shows aromatic character although its structure cannot be described as planar. An example is when benzene is cooled down to 20K so that it changes to its crystalline state and thus also change its conformation to the chair conformation. [M.Palusiak, T. M. Krygowski, 2007, Chemistry - A European Journal, Vol.13 "Application of AIM Parameters at Ring Critical Points for Estimation of pi-Electron Delocalization in Six-Membered Aromatic and Quasi-Aromatic Rings", p.7996-8006] Planarity is therefore destroyed. In its planar conformation however, its pz-orbitals (the orbitals perpendicular to the plane of the ring) merge to give a plane of electron density above and below the ring, stabilising the compound and leading to equal bond lengths between the carbons (cf MO17 shown above).
This spreading out of electron density over the ring, resembles reality. In theory, the described overlap of the pz-orbitals would lead to donut-shape-like rings of electron density above and below the molecule, leaving a hole in the middle. This is why, singly stating that aromaticity is due to the overlap of the pz orbitals is not a good method to describe the aromatic character.
Another property of aromatic compounds is the so called pi-pi stacking, in which the above mentioned planes of electron density overlap in phase or off-centre in a parallel fashion and form a layered conformation. This behaviour also results in different kinds of mixing of the pi-orbitals and therefore allows some degree of charge-transfer, as the HOMO-LUMO region of the donor and acceptor approaches in energy. [C.R. Martinez, B. L. Iverson, Chem. Sci. , 2012,"Rethinking the term "pi-stacking" " Vol.3, p.2191-2201]
Smf115 (talk) 17:29, 23 May 2018 (BST)Good discussion covering the key concepts of aromaticity and some relevant examples. To improve the MOs just visualised could be considered further, particularly with the concept of sigma-aromaticity.
Smf115 (talk) 17:29, 23 May 2018 (BST)Overall a good report which just requires further development of answers sometimes to think about other factors.






