Rep:Mod:cathyleeorganic
Module 1
Hydrogenation of Cyclopentadiene Dimer
| Compound 3 | Compound 4 | |
|---|---|---|
| Stretching | 1.2394 | 1.1034 |
| Bending | 19.1065 | 14.5151 |
| Torsion | 11.1277 | 12.5123 |
| Van der Waals | 5.8039 | 4.9856 |
| H-Bond Energy | 34.9657 | 31.1767 |
The cyclodimerisation of cyclopentadiene specifically produces compound 2. After minimising the energy of the product it was found that compound 1 has the lowest relative energy (31.8906kcal/mol cf. 34.0020kcal/mol) suggesting that it would be the thermodynamically stable compound. This therefore implies that this reaction is kinetically controlled.
Most reactions prefer to be produce the most thermosynamically stable products. Therefore the hydrogenation of dimer 2 will most likely produce compound 4 because this has the lowest energy making it the most stable compound. The bending energy is considerably lower than compound 3 which suggests that the configuration on 4 is unfavoured. Although the torsion is slightly higher for compound 4, the energy difference of the bending will outweigh this.
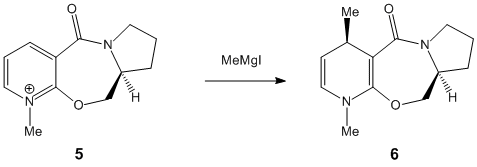
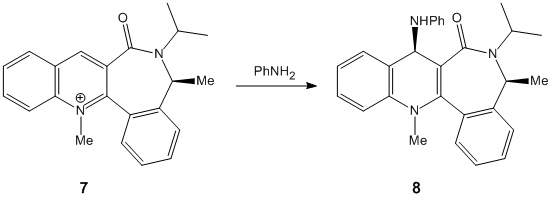
Stereochemistry of Nucleophilic Additions to a Pyridinium Ring[1]
| Compound 5 | Compound 7 | |
|---|---|---|
| Molecule | 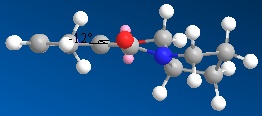 |

|
| Dihedral Angle/degrees | 11.8 | 21.1 |
Finding the most stable molecule required several tries before the lowest energy could be found. Moving the carbonyl group above and below the plane helped establish it.
Molecule 5’s carbonyl group is pointing above the plane of the ring. This means that attack of the top face is unlikely as the carbonyl group will sterically hinder the attacking MeMgI. According to the reaction scheme, the stereochemistry is correct.
Assuming that the reaction of Molecule 7 with PhNH2 , it would expected that the carbonyl group would after the sterochemistry of how the it would attack. It would attack the top face of the molecule (so the substitiuent will be in the same face as the methyl group) as the carbonyl group it pointing slightly downwards.
Intermediate in the Synthesis of Taxol

After several attempts on minimising the energy compound 10 and 11 it was found that compound 11 had the lowest energy (44.3146kcal/mol cf. 48.8879kcal/mol) and hence the most stable of the two isomer. Compound 10 has the higher energy which is probably due to the slight steric clash between the nearby hydrogens.
The alkene reacts slowly due to the extra strain the alkene is under because of it being in a cyclic ring and the hydrogens attached to the alkene. The strain energy is known as the Olefin Strain Energy[2].
Regioselective Addition of Dichlorocarbene[3]
Part One
| HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 | |
|---|---|---|---|---|---|
| Compound 12 | 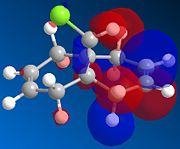 |
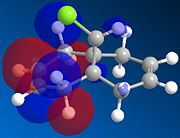 |
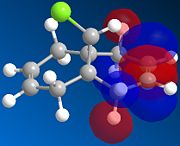 |
 |
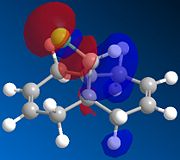
|
| Compound 13 | 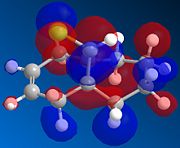 |
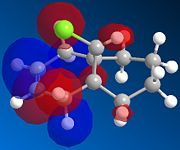 |
 |
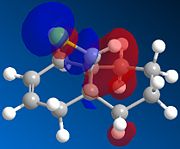 |
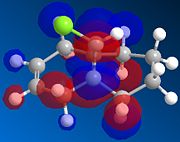
|
There is a distinct difference between the two alkenes due to the Cl being unable to rotate. There is a large probability of finding electron in the double bond nearest to the Cl, The HOMO shows that there is a large electron density aurrounding the double bond nearest to the Cl. This site therefore is the most nucleophilic and most susceptible to electrophilic attack.
The LUMO shows that for compound 12, the MO is surrounding the double bond anti to the Cl. this is where the MO are empty suggesting that nucleophiliic attack on the double bond is more likely to occur on this alkene. For compound 13 where there is only the one double bond and both the HOMO and LUMO can be found on the same alkene suggesting that both electrophiles and nucleophiles will prefer to attack there.
| Vibrational Frequency | Infrared Intensity | |
|---|---|---|
| 12 – Alkene Stretch | 1760.99 | 3.9043 |
| 12 – Alkene (anti to Cl)Stretch | 1740.89 | 4.1543 |
| 12 – Cl-C Stretch | 777.60 | 25.2299 |
| 13 – Alkene Stretch | 1761.67 | 4.2881 |
| 13 – Cl-C Stretch | 776.86 | 20.2991 |
Part Two
The Cl-C frequencies correspond to IR spectroscopy data and lies within 600-800cm-1[4]. The alkene frequencies lie slightly higher than the literature data (1620-1680cm-1). This is most likely due to the alkenes being within a ring.
When comparing the two molecules it can be seen that the results are similar except for the extra double bond in compound 12. This suggests that the alkene makes little difference the vibrational frequencies of the molecule.
Mini Project: Regio- and Stereoselective Conversion of Alkenes to Epoxides[5]
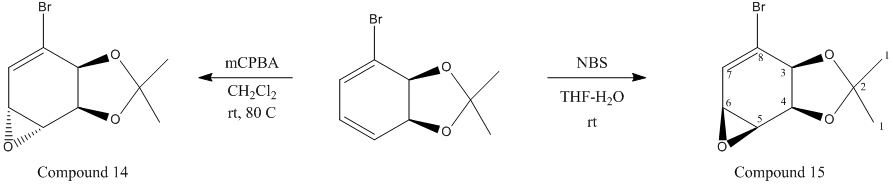
mCPBA will produce the face down epoxide because it is a bulky group and the dioxolane will be sterically clashing causing it to attack on the bottom face instead.
The best way to distinguish the two isomers is to use optical rotation. This method shines light onto the molecule and depending on the compound one will rotate a plane of polarised light clockwise (+) and one will rotate it anticlockwise (-).
| Environment | 13C Chemical Shift | Literature Chemical Shift |
|---|---|---|
| 1 | 52.0 | 25.2 |
| 2 | 140.0 | 108.7 |
| 3 | 117.8 | 76.8 |
| 4 | 115.6 | 73.9 |
| 5 | 65.5 | 27.1 |
| 6 | 80.4 | 50.2 |
| 7 | 168.7 | 125.5 |
| 8 | 170.1 | 130.0 |
| 135.6 |
13C NMR Spectroscopy
Compound 14 and 15 will have the same 13C NMR spectrum.
The results obtained do not match literature values[6] and there is an extra emvironment (there should be 8). However, most of the results obtained are shifted by around 20-30ppm when compared with the literature value.
The extra chemical shift obtained could correspond with environment 2 but it will be hard to tell which one is which.
IR Spectroscopy
| Frequency/cm-1 | Corresponds to | Intensity |
|---|---|---|
| 3149.41 | C-H Stretch | 39.68 |
| 1710.37 | C=C Stretch | 30.56 |
| 1266.25 | C-C Stretch | 154.63 |
| 1106.66 | C-O Stretch | 195.96 |
| 584.41 | C-Br Stretch | 38.47 |
The IR frequencies match the literature data[7] for an IR spectrum. Because the two molecules are stereoisomers they have nearly the same IR spectrum (±50cm-1).
The free energy calculated for both molecules was found to have the same value at -3146.75 Hartrees.
Optical Rotation
As predicted one isomer has a positive and negative value of [α]D. Compound 14 has a value of 93.23 degrees and compound 15 has a a value of -101.78 degrees. Literature value show that compound 15 should have -76.2 degrees[8]. Although the number is not exact, the most important thing is that the rotation of light is the same.
References
- ↑ Regio- and stereoselective control in the addition of Grignard reagents to the pyridine ring system: DOI:10.1021/jo00356a016
- ↑ Tetrahedron Volume 53, Issue 28, 14 July 1997, Pages 9727-9734 DOI:10.1016/S0040-4020(97)00595-4
- ↑ J. Chem. Soc., Perkin Trans. 2, 1992, 447 - 448DOI:10.1039/P29920000447
- ↑ Characteristic IR Absorption Frequencies of Organic Functional Groups http://www2.ups.edu/faculty/hanson/Spectroscopy/IR/IRfrequencies.html
- ↑ Tetrahedron: Asymmetry Volume 16, Issue 7, 4 April 2005, Pages 1393-1402DOI:10.1016/j.tetasy.2005.02.012
- ↑ Tetrahedron Volume 53, Issue 26, 30 June 1997, Pages 8807-8814 DOI:10.1016/S0040-4020(97)90392-6
- ↑ Characteristic IR Absorption Frequencies of Organic Functional Groups http://www2.ups.edu/faculty/hanson/Spectroscopy/IR/IRfrequencies.html
- ↑ Tetrahedron Volume 53, Issue 26, 30 June 1997, Pages 8807-8814 DOI:10.1016/S0040-4020(97)90392-6